Review Article
Consideration on the Water Contamination of Cyanobacterial Extracellular Polymeric Substances (EPS)
Feng Sun1*, Rong Pan1, Aijuan Qian1, Haibing Cong1 and Gisella R Lamas-Samanamud2
1School of Environmental Science and Engineering, Yangzhou University, Yangzhou, Jiangsu, China
2Department of Civil and Environmental Engineering, University of Texas at San Antonio, San Antonio, Texas, USA
*Corresponding author: Feng Sun, School of Environmental Science and Engineering, Yangzhou University, Jiangsu 225127, PR China, Tel: +86 51487978626; Fax: +86 51487978626; E-mail: sunfeng@yzu.edu.cn
Citation: Sun F, Pan R, Qian A, Cong H, Lamas-Samanamud GR (2017) Consideration on the Water Contamination of Cyanobacterial Extracellular Polymeric Substances (EPS). J Environ Sci Allied Res 2017: 19-24. doi:https://doi.org/10.29199/2637-7063/ESAR-101013
Received Date: 26 July, 2017; Accepted Date: 18 September, 2017; Published Date: 03 October, 2017
Abstract
Cyanobacterial Extracellular Polymeric Substances (EPS), a protective barrier between the cells and the external environment, is an organic matter with a high molecular viscosity which is formed and secreted during the growth and reproduction of cyanobacteria cells. The investigation of EPS contributes to a better understanding of the growth and proliferation of cyanobacteria. Although some significant research includes the ecological significance and industrial application of EPS, the influence of EPS on the water environment and water quality have not been fully recognized. Based on a large amount of literature review and live survey on the cyanobacterial blooms, this paper summarizes an overview of the water contamination of cyanobacterial EPS. Future research directions should focus on the analysis of the contamination mechanism of cyanobacterial EPS and its control methods.
Keywords: Contamination; Control; Cyanobacteria; EPS
Formation and Secretion of Cyanobacterial EPS
In recent years, water environmental problems have become increasingly serious. A wide concern is given to cyanobacterial blooms which incidence seems to be frequent due to the eutrophication of water bodies. Cyanobacteria are aquatic and photosynthetic, which grow in colonies and reproduce rapidly. Researchers found that the formation and secretion of Extracellular Polymeric Substances (EPS) were accompanied by the growth of many species of cyanobacteria, such as Microcoleus [1,2] Anabaena [3], Microcystis [4], Nostoc [5] and so on. The EPS works as a protective barrier between the cell and the external environment which allows the microorganism or the biofilm formed to be resistant to drought, ultraviolet radiation, biological mineralization, and protozoan predation [6,7]. The protective function of the EPS is closely related to its components, which is essentially a combination of natural organic molecules, including polysaccharides, proteins, nucleic acids, phospholipids, uronic acids, alginic acids, and humic acids. Polysaccharides, including the main monosaccharides such as glucose, galactose, xylose and rhamnose, and the carbon-containing and nitrogen-containing proteins primarily account for about 70% - 80% of its composition [2,8,9].
The formation of EPS is influenced by the combined effects of cellular activities and environmental factors. Rao et al., [10] studied the effects of temperature and salinity on the EPS yield of Scytonema javanicum and indicated that in a certain range, the EPS yield would increase with the increase of temperature and salinity. Chen et al., [11] found that pH <8 would promote the accumulation of EPS of Cylindrotheca closterium. Ge et al., [12] found EPS yield of Nostoc increased with the light intensity. In addition, continuous light and high-light intensity leads to an increase in protein content in the EPS without affecting the monosaccharide content. This suggests that light intensity affects both the yield and the distribution of EPS components. Svane and Eriksen [13] analyzed the kinetics of the EPS synthesis of Microcystis flos-aquae. In this case, EPS had a higher synthesis rate up to 76 mg/(g·day) during the exponential growth phase and a six times lower rate of 12 mg/(g·day) during stationary phase, which indicated that EPS growth rate is closely related to the amount of nutrients available in the system. Different C and N sources were used in the comparison of cell growth and EPS production of Aphanothece halophytica. Nitrogen in the form of nitrate has been found to be the best nitrogen source for both cell and EPS growth. CO2 in the atmosphere was the best carbon source for the algal cell growth, while sodium acetate was the best for the EPS production [14]. The increase in EPS formation was related to the C/N ratio [15]. Chen et al., [16] found that the heavy metal Cd2+ could significantly inhibit the growth of algal cells and their EPS, thus, causing different distribution of attached EPS and colloidal EPS in different growth stages. Furthermore, the study of co-culture and fermentation of Cyanobacteria, Chlorella, and Basidiomycetes, revealed that fungus could significantly stimulate the generation of cyanobacterial EPS [17]. Therefore, the EPS is widespread in the cyanobacteria-containing environment and its formation and secretion is not only restricted by the characteristics of different algal species but also by the influence of pH, light intensity, temperature, salinity, nutrients, growth time and other environmental factors [12,18,19].
Water Contamination of Cyanobacterial EPS
Contamination of water sources by cyanobacterial EPS
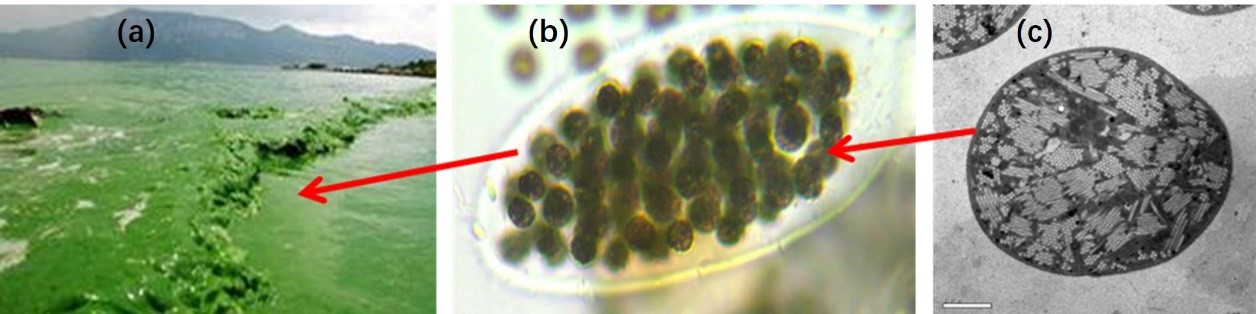
Cyanobacteria grow and reproduce rapidly in eutrophic water and float upward on the surface of water due to the buoyancy produced by gas vesicles. The EPS wrapped on the surface of cyanobacterial cells attach hundreds or thousands of cells with a diameter of 4~6 μm together and form amorphous aggregated particles [9,13,20]. With the formation and secretion of EPS, the aggregated cyanobacterial particles continue to expand until they cover the entire surface of the water, which will cut off the water body with air (Figure 1). And the growth and reproduction of cyanobacterial cells will consume a large amount of dissolved oxygen that affects the ecological environment of the water. In addition, most EPS released by cyanobacteria is mainly hydrophilic organic matters with a large number of carboxyl, hydroxyl, and amino groups, which greatly increases the content of dissolved organic matters and aggravates the contamination of water bodies [21,22]. Besides, the components of proteins and humic acids containing carbon and nitrogen in the cyanobacterial EPS would produce a large number of carbonaceous and nitrogenous derivatives due to the natural oxidation or degradation which are carcinogenic remaining in water [23]. This limits the other application of cyanobacterial EPS and is undoubtedly an incidental ecological disaster on the water environment.
|
Figure 1: Cyanobacterial aggregates attached by cyanobacterial EPS. (a) cyanobacterial particles float and expand to form cyanobacterial bloom; (b) EPS attach cells into cyanobacterial particles [24]; (c) the slice image of cyanobacterial cell [22]. |
Influence of cyanobacterial EPS on water treatment processes
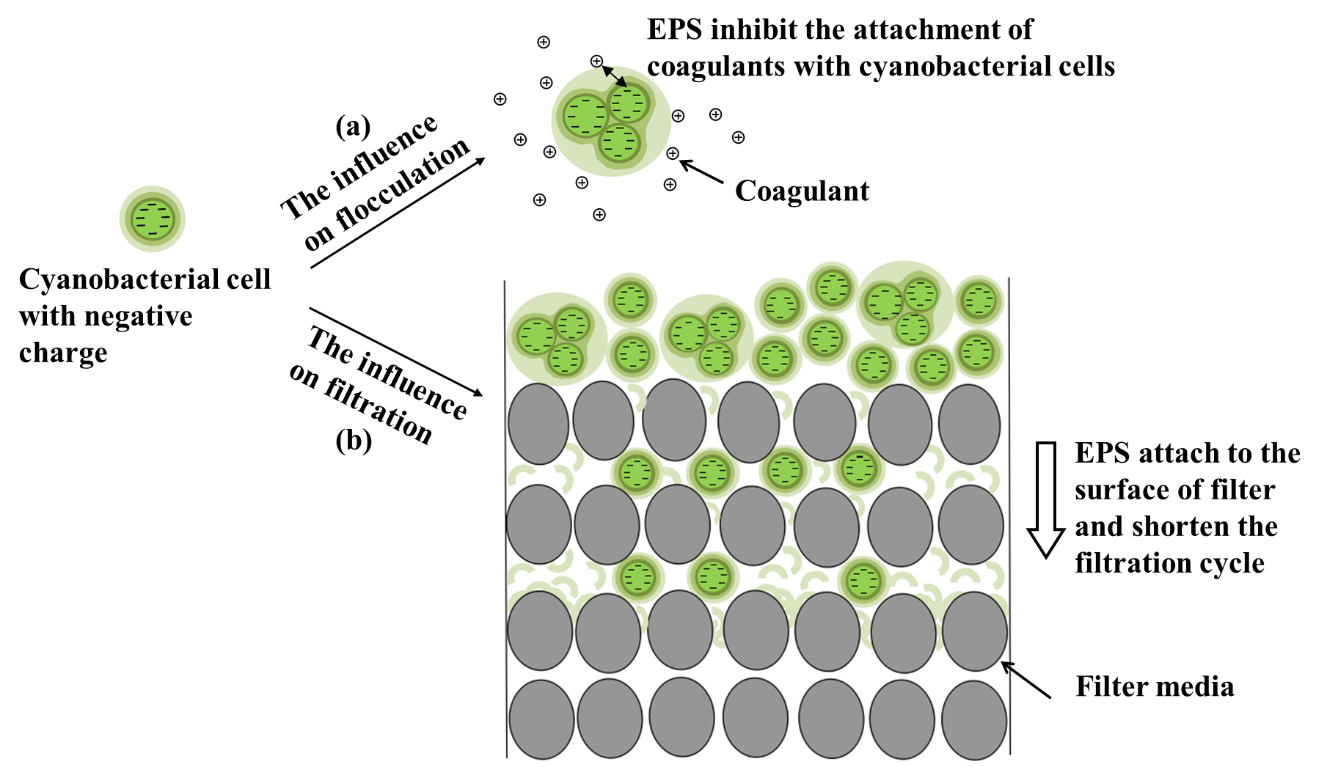
Not only cyanobacterial cells, but the presence of a large amount of EPS is also a technical problem in water treatment project. Cyanobacterial EPS is difficult to remove in conventional water treatment processes, because it is easy to wrap in the surface of small inorganic particles becoming the source of organic colloids. Coagulation is the key process for cyanobacteria removal in conventional drinking water treatments. The coagulants will adsorb both cyanobacterial cells and EPS to form insoluble complexes which increase the density of cyanobacterial cells and promote the sedimentation necessary for cyanobacteria removal. Studies have shown that the removal efficiency of monocellular discrete cyanobacterial cells without EPS could reach up to 80%~90% through the coagulation process [25,26]. However, for aggregated cyanobacterial particles, their secreted EPS having characteristics of anionic polymer with negative zeta potential in the pH range of 2~10 [27] and attaching to the surface of the colloidal cells, would increase the stability of colloids and reduce the coagulation efficiency (Figure 2a). Besides, parts of the EPS components have negative effects on flocculation, clarification, sedimentation and sludge dewatering. Large amounts of EPS will weaken the cell adhesion, deteriorate the floc structure, aggravate the cell erosion and reduce the sludge dewatering effect [28]. For instance, the acidic substances in EPS will react with the hydrolysis products of coagulants. The generated surface complexes will attach to the surface of the floc particles hindering the collision of particles and forming flocs with low density and bad sedimentation effect. This leads to an increase dosage of coagulant to compensate the influence of formed surface complexes on the destabilization and flocculation of particles [29].
|
Figure 2: Influence of cyanobacterial EPS on, (a) coagulation, and (b) filtration process. |
Filtration is a physical separation process for large single cells and aggregated algal particles. During the cyanobacteria removal and separation process, the secreted EPS will attach to the surface of the filter and form biofilm to shorten the filtration cycle (Figure 2b). It also affects the filtration efficiency of cyanobacteria-containing water. During the initial and mid-term of filtration, the interception of cyanobacterial cells and organic matters will obviously reduce the content of pollutants in the filtered water. But during the later period, the cyanobacterial cells will slowly penetrate the filter and the trapped organic matters will gradually desorb resulting in the increase of the organic content of the water [29].
Threats of cyanobacterial EPS to water supply
People pay attention to the safety of water quality gradually rising from treated water to supplied water. The investigation of the water quality of 45 cities in China showed that the qualification rate of water quality fell by nearly 20% after they were transferred out of the treatment plant and passed through the water supply network, of which the conventional microbial indicator of total bacteria increased nearly 4 times [30,31]. The microbes would like to grow on the surface of water pipe and accumulate into biofilm with EPS in a long-term contact with water. The organic components of cyanobacterial EPS would also provide sufficient carbon sources for microbes and promote their reproduction. Therefore, the residual cyanobacterial EPS remained in water will greatly promote the growth of biological membrane in pipe network. As we know, the removal efficiency of organic contaminants, such as cyanobacterial EPS, is relatively low for most traditional water treatment processes, a large amount of residual EPS remain in water as organic nutrients to promote the growth of bacteria in pipe network, even if using high residual chlorine, bacteria can still exist in the distribution network [32]. Therefore, the residual EPS and their organic derivatives are important factors restricting the water quality and biological stability of water supply network, which will directly threaten the safety of drinking water.
The Control Mechanisms of Cyanobacterial EPS
At present, the cyanobacterial EPS as a kind of organic contaminant, has not attracted enough attention, and their regulation and degradation has not yet effectively carried out. A study showed that the aggregation ability of cyanobacteria decreased by 27.6-57.4% after the extraction of EPS [9], which indicates that the removal of EPS would weaken the population growth of cyanobacteria and play an important role in preventing cyanobacterial bloom. Therefore, the regulation and degradation of cyanobacterial EPS have important guiding significance for the effective control of cyanobacteria pollution.
The removal of cyanobacterial EPS with physical methods
The common physical method used for EPS removal is centrifugation, but other physical and chemical auxiliary forms are often needed during the centrifugation. Flaibani et al., [33] successfully realized the efficient removal of cyanobacterial extracellular polysaccharides by the high-speed centrifugation with 10000 g; Su et al., [34] realized effective separation of soluble and bonded EPS of cyanobacteria by the low-speed centrifugation combined with 80oC water bath method; and Xu et al., [9] using variable-speed centrifugation combined with buffer extraction and 60oC water bath method achieved the step-by-step removal different binding-state EPS.
In the process of centrifugation for EPS removal, attention should be paid to the coordination of centrifugation force and removal efficiency, avoiding the breakage of cyanobacterial cells and the leakage of endotoxins. And in the action of auxiliary forms, there are problems of protein denaturation caused by high temperature and cyanobacteria rupture caused by chemical substances, therefore, the temperature of water bath, the composition of buffer solution and other conditions should also be investigated.
Ultrasonic pretreatment could destroy the gas vesicles inside of the cyanobacterial cells and thus, increase the sedimentation of cyanobacteria. However, the low-power ultrasonic treatment would depolymerize the cyanobacterial groups, discrete the cyanobacterial cells and weaken the adverse effects of EPS. High-power ultrasonic treatment and long duration time would easily lead to the lysis of cyanobacterial cells and the additional release of intracellular toxins [35-37]. Therefore, the conditions for the implementation of ultrasonic method are still questionable.
The activated carbon possesses micro-pore structure and many functional groups such as hydroxyl radicals on the surface, which could be easy to combine with the cyanobacterial EPS and remove the organic matters [38]. However, the influence of cyanobacterial cells and EPS on activated carbon technology is mainly reflected on the adsorption saturation period with a high concentration of cyanobacteria. The high presence of EPS will reduce the adsorption effect of activated carbon and result in the bacteria breeding. Besides, due to the athletic ability and applicability of the cyanobacterial cells, parts of the cyanobacteria will continue to grow in the high organic concentration on the surface of activated carbon, which need to promptly carry out the desorption of activated carbon [29].
The application of membrane separation technology in the purification of drinking water has developed rapidly in recent years and is considered to be the most promising advanced water treatment technology. It mainly filters out algae, particles and other organic pollutants like EPS by physical methods. Water assays from ultrafiltration membrane treatment with high concentration of algae showed that the ultrafiltration membrane had high removal and retention efficiency for algae, bacteria, and turbidity [39]. Removal efficiency of membrane with pore size of 30 μm could reach to more than 98% but the destruction rate was less than 2% [40]. Nonetheless, membrane fouling is easily caused at high algae period. The cyanobacterial cells and EPS that remained on the surface of the membrane could adhere to organic particles, increase the transmembrane pressure and result in the dramatic reduction of flux [41]. Therefore, the large amount of cyanobacterial EPS has greatly increased the difficulty of membrane separation and reduced the efficiency of membrane treatment. The adverse effects of membrane separation cannot be underestimated and the application of this technique is difficult.
The degradation of cyanobacterial EPS with chemical methods
The pre-oxidation is relatively established and effective pretreatment for the control of cyanobacterial bloom. Chlorine pre-oxidation, ozone pre-oxidation, and potassium permanganate pre-oxidation are widely used to oxidize the EPS and enhance the coagulation efficiency of cyanobacteria [42,43]. In comparison, chlorine is a strong oxidant which can degrade most of the EPS rapidly at lower oxidant dose, however, it also has a strong ability to kill cyanobacteria and is more likely to destroy the cell structure and reduce cell activity. While potassium permanganate as a better choice, its performance of pre-oxidation is influenced by factors such as morphology, motility and EPS content of cyanobacterial cells. EPS adheres to the surface of cyanobacterial cells, which would weaken the oxidation and consume more pre-oxidants. But an excessive dose of pre-oxidants would lead to the disruption of cyanobacterial cells and the release of intracellular toxins [13]. Therefore, the way of chemical pre-oxidation still needs to find a coordinated treatment to keep the degradation efficiency and ensure the cell integrity.
The residual EPS and other organic components of water rafter filtration can still be degraded by disinfection process. However, the potential threats of disinfection by-products should be considered. It was found that EPS was easy to react with chlorine and produced chlorinated disinfection by-products, accounting for 63% of the trihalomethane production potential and 31.25% of the haloacetic acid production potential, which affected the quality of treated water [23,44]. Besides, a large amount of nitrogen-containing organic matter can be easily converted into nitrogen-containing disinfection by-products during disinfection, while the nitrogen-containing disinfection by-products are more toxic [23]. Therefore, as an important organic contamination source and the main precursor of various disinfection by-products, the secondary pollution of cyanobacterial EPS is the most important problem in the process of chemical degradation.
The control of cyanobacterial EPS with microbial methods
For a long time, the regulation of EPS mostly starts with physical and chemical methods, while few focuses on the biodegradation with the action of microorganisms. By now, only Colombo [45] used glycosidases from heterotrophic microorganisms of Barra Bonita integrated water sample for the degradation of EPS produced by Anabaena spiroides and found that the EPS could be totally consumed by the glycosidases, because the bacterial population was able to grow using EPS as a carbon source. The EPS degradation was found to occur in a two-phase process. The first consisted of high enzymatic activity that consumed 41% of the EPS at a relatively high rate, while the second consumed the remaining 59% at a slower rate. But the microbial action took longer time and the enzymatic hydrolysis period was about 29 days. Besides, this study ignored the pollution caused by the heterotrophic microorganisms, thus even if it is feasible to control cyanobacterial EPS by the method of microbial degradation, the water contamination due to microbial factors should not be underestimated. In addition, the degradation bacteria is anaerobic and it must be operated under strict condition without oxygen, which will bring about some new problems such as water body deterioration. By now, the microbial method is still a controversial topic. Many countries, especially the developed countries, are particularly careful to the use of microbial method, in order to avoid a series of problems of biological invasion, ecological balance destruction, etc. Therefore, it is still necessary to evaluate comprehensively whether the microbial regulation can be an effective method for cyanobacterial EPS control, and the accuracy of objectives and operating costs still need investigation.
Prospects
The formation and secretion of cyanobacterial EPS have important influences on the integrity of cyanobacterial cells, the contamination of cyanobacterial blooms and the performance of coagulation during drinking water treatment processes. Currently, there are many researchers around the world focusing on various methods and strategies for the regulation and control of cyanobacteria contamination. Many drinking water plants also carry out the optimization and adjustment of water treatment processes for cyanobacteria contaminated water sources. However, control strategies mainly aim at the elimination of cyanobacterial cells and the degradation of microcystins, but the regulation of cyanobacterial EPS is still insufficient. Therefore, a reasonable regulation of shedding and degradation of cyanobacterial EPS becomes a technical problem of water treatment processes. Based on the above analysis, the most urgent problems during the cyanobacterial EPS studies are as follows: (1) cyanobacterial EPS as a high organic content contamination source, its contamination mechanism and control method are not clear; (2) the extracellular wrapping of EPS hinders the combination of cyanobacterial cells with flocculants, which reduces the performance of flocculation and other water treatment processes; (3) the residual cyanobacterial EPS has become an important contributor to bacteria breeding in the pipe network, and there is still no safe and efficient EPS degradation technology. Therefore, the future research should focus on the comprehensive and in-depth contamination mechanism analysis and reasonable control methods of cyanobacterial EPS.
Acknowledgments
The authors acknowledge financial supports from National Natural Science Foundation of China (51708480), Natural Science Foundation of Jiangsu Province (BK20150456), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (15KJD610006), Yangzhou Key Research Project of Social and Development (YZ2015072), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Science and Technology Innovation Fund Project of Yangzhou University (2015CXJ036).
References
- Vicente-García V, Ríos-Leal E, Calderón-Domínguez G, Cañizares-Villanueva RO, Olvera-Ramírez R (2004) Detection, isolation, and characterization of exopolysaccharide produced by a strain of Phormidium 94a isolated from an arid zone of Mexico. Biotechnology and Bioengineering 85: 306-310.
- Richert L, Golubic S, Guédès RL, Ratiskol J, Payri C, et al. (2005) Characterization of exopolysaccharides produced by cyanobacteria isolated from polynesian microbial mats. Curr Microbiol 51: 379-384.
- Obst M, Dynes JJ, Lawrence JR, Swerhone GDW, Benzerara K, et al. (2009) Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochimica et Cosmochimica Acta 73: 4180-4198.
- Foster JS, Green SJ, Ahrendt SR, Golubic S, Reid RP, et al. (2009) Molecular and morphological characterization of cyanobacterial diversity in the stromatolites of Highborne Cay, Bahamas. ISME J 3: 573-587.
- Otero A, Vincenzini M (2003) Extracellular polysaccharide synthesis by Nostoc strains as affected by N source and light intensity. J Biotechnol 102: 143-152.
- Böhm GA, Pfleiderer P, Böger P, Scherer S (1995) Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J Biol Chem 270: 8536-8539.
- Lin CS, Wu JT (2014) Tolerance of soil algae and cyanobacteria to drought stress. J Phycol 50: 131-139.
- Wang W, Huang HD, Zhang Y, Ma T, Zhang GP, et al. (2008) Rheological and gelling properties of a novel biopolymer Ss. Microbiology 35: 866-871.
- Xu H, Jiang H, Yu G, Yang L (2014) Towards understanding the role of extracellular polymeric substances in cyanobacterial Microcystis aggregation and mucilaginous bloom formation. Chemosphere 117: 815-822.
- Rao BQ, Zhang JY, Lou YM, Mei HX, Wang YL, et al. (2015) Effects of different environmental factors on the extracellular polysaccharide exudation of Scytonema javanicum. Journal of Xinyang Normal University (Natural Science Edition) 28: 496-500.
- Chen CP, Gao YH, Lin P (2006) Production of Extracellular Polymeric Substances (EPS) by benthic diatom: effect of salinity and pH. Acta Oceanologica Sinica 28: 123-129.
- Ge H, Xia L, Zhou X, Zhang D, Hu C (2014) Effects of light intensity on components and topographical structures of extracellular polysaccharides from the cyanobacteria Nostoc sp. J Microbiol 52: 179-183.
- Svane R, Eriksen NT (2015) Exopolysaccharides are partly growth associated products in Microcystis flos-aquae. Journal of Applied Phycology 27: 163-170.
- Zhu FY, Li H, Wei P (2002) Cell growth rate and exopolysaccharides product by aphanothece halophytica under different nutrition. Journal of Nanjing University of Technology (Natural Science Edition) 24: 57-59.
- Zhang CS, Wu J (1997) Review on research of cyanobacteria extracellular polysaccharide. Chinese Journal of Marine Drugs 3: 20-25.
- Chen CP, Xu HL, Liang JR, Gao YH (2013) The influence of Cd2+ on the extracellular polymeric substances excreted by the benthic diatom cylindrotheca closterium from mangrove. Journal of Xiamen University (Natural Science) 52: 122-126.
- Angelis S, Novak AC, Sydney EB, Soccol VT, Carvalho JC, et al. (2012) Co-culture of microalgae, cyanobacteria, and macromycetes for exopolysaccharides production: process preliminary optimization and partial characterization. Appl Biochem Biotechnol 167: 1092-1106.
- Bi YH, Hu ZY (2004) Influence of temperature, nutrients and light intensity on the growth of Nostoc flagelliforme. The Chinese Journal of Process Engineering 4: 245-249.
- Lei LM, Song LR, Ou DY, Han BP (2007) Effects of nutrient conditions on exopolysaccharide production in water-bloom forming cyanobacteria, Microcystis aeruginosa. Acta Scientiarum Naturalium Universitatis Sunyatseni 46: 84-87.
- Hu HJ (2011) Cyanobacteria Biology. Science Press, Beijing, China.
- Ghernaout D, Moulay S, Messaoudene NA, Aichouni M, Naceur MW, et al. (2014) Coagulation and chlorination of NOM and algae in water treatment: A review. International Journal of Environmental Monitoring and Analysis 2: 23-34.
- Jiang XY, Liu YJ, Cong HB, Zhu XY, Xu ST (2014) Control of disinfection byproducts in cyanobacteria containing water treatment by pressurized coagulation and sedimentation. Chinese Journal of Environmental Engineering 9: 1763-1770.
- Fang J, Ma J, Yang X, Shang C (2010) Formation of carbonaceous and nitrogenous disinfection by-products from the chlorination of Microcystis aeruginous. Water Res 44: 1934-1940.
- Sun XX, Cong HB, Gao ZJ, Cui CJ, Cao QQ (2014) Movement characteristics of cyanobacteria under stress of water-lifting aeration. Environmental Science 35: 1781-1787.
- Sun F, Pei HY, Hu WR, Ma CX (2012) The lysis of Microcystis aeruginosa in AlCl3 coagulation and sedimentation processes. Chemical Engineering Journal 193-194: 196-202.
- Sun F, Pei HY, Hu WR, Li XQ, Ma CX, et al. (2013) The cell damage of Microcystis Aeruginosa in PACl coagulation and flocstorage processes. Separation and Purification Technology 115: 123-128.
- Henderson RK, Baker A, Parsons SA, Jefferson B (2008) Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms, Water Res 42: 3435-3445.
- Li XY, Yang SF (2007) Influence of loosely bound Extracellular Polymeric Substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Research 41: 1022-1030.
- Wang N, Ge F, Wu XZ, Zhu RL, Zhu MJ (2010) Effect of algae and its extracellular organic matter on drinking water treatment. Technology of Water Treatment 36: 19-24.
- Zhang MD, Cai YL, Bai XH (2010) Microbial safety of water and its evaluation in city water supply system in China. Water & Wastewater Engineering 36: 30-33.
- Tong ZG, Liu SQ (2005) Protection measures for the safety of water quality of distribution system. Water Purification Technologies. 24: 49-53.
- Zhang Q, Yang YL, Li X, Cui CW, Ji F, et al. (2005) Study on different indicators of controlling biological stability in drinking water supply system. Journal of Harbin University Commerce (Natural Science Edition). 21: 30-34.
- Flaibani A, Olsen Y, Painter TJ (1989) Polysaccharides in desert reclamation: Compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydrate Research 190: 235-248.
- Su JY, Jia SR, Chen XF, Yu HF (2008) Morphology, cell growth, and polysaccharide production of Nostoc flagelliforme in liquid suspension culture at different agitation rates. Journal of Applied Phycology 20: 213-217.
- Zhang GM, Zhang PY, Wang B, Liu H (2006) Ultrasonic frequency effects on the removal of Microcystis aeruginosa. Ultrasonics Sonochemistry 13: 446-450.
- Liang H, Nan J, He WJ, Li GB (2009) Algae removal by ultrasonic irradiation-coagulation. Desalination 239: 191-197.
- Wu XG, Joyce EM, Mason TJ (2012) Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Research 46: 2851-2858.
- Ren ZJ, Ma GL, Liu GF, Ma J (2011) Effect of the combined process of permanganate preoxidation-BAC filtration on the removal of algae. Industrial Water Treatment 31: 55-57.
- Zhao H (2008) Study on algae removal technique source water by ultrafiltration process. Beijing Industry University, Beijing, China.
- Gijsbertsen-Abrahamse AJ, Schmidt W, Chorus I, Heijman SGJ (2006) Removal of cyanotoxins by ultrafiltration and nanofiltration. Journal of Membrane Science 276: 252-259.
- Teixeira MR, Rosa MJ (2005) Microcystins removal by nanofiltration membranes. Separation and Purification Technology 46: 192-201.
- Chen JJ, Yeh HH, Tseng IC (2009) Effect of ozone and permanganate on algae coagulation removal - Pilot and bench scale tests. Chemosphere 74: 840-846.
- Ma M, Liu RP, Liu HJ, Qu JH, Jefferson W (2012) Effects and mechanisms of pre-chlorination on Microcystis aeruginosa removal by alum coagulation: Significance of the released intracellular organic matter. Separation and Purification Technology 86: 19-25.
- Luo JH, Fu Q, Zheng BH, Zhao XR, Liu Y, et al. (2012) The composition of DOM during water bloom and their disinfection by-products formation potential. Journal of Basic Science and Engineering 20: 210-218.
- Colombo V, Vieira AAH, Moraes G (2004) Activity of glycosidases from freshwater heterotrophic microorganisms on the degradation of extracellular polysaccharide produced by Anabaena spiroides (Cyanobacteria). Brazilian Journal of Microbiology 35: 110-116.
 LOGIN
LOGIN REGISTER
REGISTER.png)