Research Article
Tyrosinase inhibitors with potent anti-senescence activity in human neonatal keratinocyte progenitors
Short running title: Decapeptide-12 modulates sirtuin gene expression
Anan Abu Ubeida, Basil M Hantash*a,b
aEscape Therapeutics, Inc. USA.
bEscape Therapeutics, Inc. 3800 Geer Road, Suite 200, Turlock, CA 95382, USA.
*Corresponding Author : Basil M Hantash, Escape Therapeutics, Inc. 3800 Geer Road, Suite 200, Turlock, CA 95382, USA. E-mail : basil@escapetherapeutics.com
Citation : Basil MH, Anan AU (2019) Tyrosinase inhibitors with potent anti-senescence activity in human neonatal keratinocyte progenitors. J Dermatol Surg Res Ther, 2019: 30-39.
Received Date: 05 October, 2018; Accepted Date: 03 January, 2019; Published Date: 21 January, 2019
Abstract
Background: Sirtuins exhibit pleiotropic effects on premature aging, cellular senescence, longevity, and a wide range of aging disorders.
Aim: We assessed effects of decapeptide-12, tyrosinase inhibitor, on sirtuin gene expression levels in human neonatal keratinocyte progenitors.
Methods: We quantitated effects of decapeptide-12 on 7 sirtuin genes using RT-PCR and its effects on cellular viability and proliferation after 72-h incubation with various concentrations of decapeptide-12 and oxyresveratrol.
Results: 100 µM decapeptide-12 increased transcription of SIRT1 by 141 ± 11% relative to control cells, whereas levels of SIRT3, SIRT6, and SIRT7 were increased by 121 ± 13%, 147 ± 8% and 95 ± 14%, respectively.
Conclusion: Decapeptide-12 upregulated sirtuin transcription to similar levels as oxyresveratrol but with reduced cytotoxicity.
Key words: decapeptide-12; oxyresveratrol; sirtuins; anti-senescence; anti-aging proteins; tyrosinase inhibitors; hypopigmentation; anti-inflammation.
Introduction
Skin manifests the consequences of chronological and photoaging rendering us constantly aware of the aging process and seeking remedies to slow or reverse its impact. Skin aging has traditionally been categorized as extrinsic or intrinsic [1,2]. Recent evidence indicates that both types are characterized by connective tissue damage [3], reduced procollagen synthesis, and enhanced matrix metalloproteinase expression [4].
Aging of human skin results in diminished cellular proliferation and differentiation and increased senescent cells [5-7]. These alterations result from free radical damage by various native reactive oxygen species (ROS) [8]. In vivo data further supports this theory as linkage between skin aging and cellular senescence were shown to result from mitochondrial oxidative damage [9]. Furthermore, mutagenesis and photoaging resulted from ROS triggered by ultraviolet (UV) radiation [10,11]. Consistent with these data, UV exposure also altered sirtuin expression supporting the importance of sirtuins in skin aging [12,13].
Cellular senescence involves arrest of cell division with resultant specific phenotypic changes such as altered chromatin and secrotome coupled with tumor-suppressor activation [14-16]. Numerous reports helped establish sirtuins as potent anti-aging proteins orchestrated via modulating pathways involving telomere length, DNA repair, and oxidative stress [17-23].
Seven sirtuin genes (SIRT1–7) are present in mammals, each with unique localization and activity [24,25]. Sirtuins maintain NAD+ dependent lysine deacetylase activity [18,26] and serve as important metabolic regulators, cellular energy and redox sensors, and oxidative stress modulators [27,28] .
These findings have triggered interest in developing small molecule activators or pharmaceuticals to help slow the progression of aging and its wide range of age-associated disorders. The effects of SIRT1 on aging and longevity have been thoroughly studied. For instance, resveratrol, a well-known anti-aging nutraceutical, derives its anti-aging effects via SIRT1 activation [29,30] and upregulation of AMP-activated protein kinase activity to diminish cellular senescence and proliferative dysfunction [31] .
We have previously reported on decapeptide-12's potent hypopigmenting efficacy in human skin [32]. Further clinical studies revealed an overall improvement in facial skin appearance in patients with dyschromia who were treated twice daily with topical cream containing 0.01% decapeptide-12 for 8 weeks [33,34]. These findings led us to hypothesize that decapeptide-12 may modulate sirtuin activity to improve overall skin appearance. To clarify this possibility, we assessed the effects of decapeptide-12 on sirtuin transcription in human neonatal keratinocyte progenitors.
Materials and methods
Chemicals
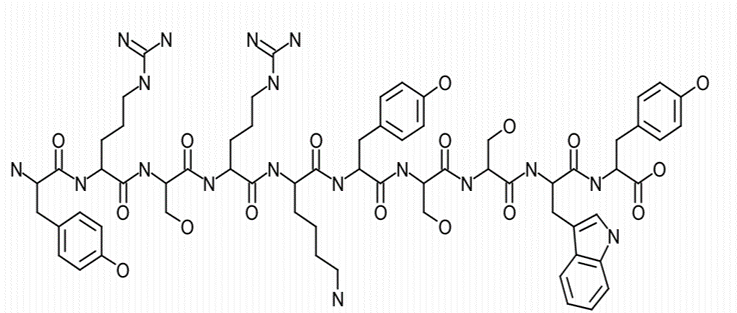
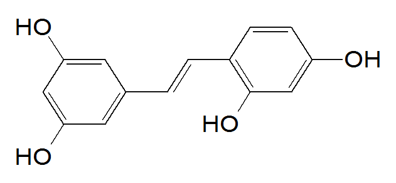
Solid-phase FMOC chemistry was utilized to synthesize decapeptide-12 (YRSRKYSSWY; Figure. 1A) (Bio Basic, Inc., Ontario, Canada) [32]. Oxyresveratrol (Figure. 1B) and all other chemicals used were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
|
A
B Figure 1. Chemical structure of decapeptide-12 (A) and oxyresveratrol (B). |
Cell culture
Human neonatal keratinocyte progenitors (Thermo Fisher Scientific, NY) were seeded in 6-well plates at a density of 2×105 cells/well. Each well received 2 ml of Epilife media containing 60 µM calcium chloride (Thermo Fisher Scientific, NY). Plates were incubated in a humidified chamber at 37ºC and 5% CO2. Twenty-four hours later, cells were treated with various concentrations of oxyresveratrol or decapeptide-12 dissolved in PBS containing 5% DMSO. Control wells received vehicle only (5% DMSO and PBS). Final concentration of DMSO in each well was 0.05%.
Total RNA extraction, quantitation, and cDNA synthesis
After a 72-h incubation period, cells were trypsinized and total RNA extracted using RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. RNA concentration was determined using nanodrop (Thermo fisher scientific, NY). Two µg of total RNA were used to synthesize cDNA using oligo dT primers and TaqMan reverse transcription reagents (Thermo fisher scientific, NY). A DNA Engine Peltier Thermal Cycler (Bio-Rad, Hercules, CA) was used to carry out reactions as follows: annealing at 25 ºC for 10 min, first strand synthesis at 48 ºC for 1 h, and heat inactivation at 95 ºC for 5 min.
Semi-quantitative analysis
SIRT1-7 primers (table 1) were designed using Primer3 [35]. Semi-quantitative PCR reactions were performed on a DNA Engine Peltier Thermo Cycler (Bio-Rad, Hercules, CA). PCR was carried under the following conditions: denaturation at 94 ºC for 2 min and primer extension at 54 ºC for 30 s in 34 cycles for SIRT 1-7 and the housekeeping gene 18S.
Table 1. Primer sequences for SIRT 1-7 and 18S. |
Samples were run and resolved on a 1.5% agarose gel containing 0.5 µg/ml of ethidium bromide and imaged using the FluorChem HD2 Imaging System (Protein simple, San Jose, CA). AlphaEase FC software (Protein simple, San Jose, CA) was used to carry out densitometry analysis. Intensity value for each gene divided by the intensity value of the internal control gene 18S represented the intensity ratio.
Viability/proliferation and cytotoxicity assays
TACS® MTT Cell Proliferation Kit (R&D systems, Minneapolis, MN) was used to determine proliferation rates. Cells were seeded at 2.5 × 104/well in 96-well plates in a humidified atmosphere with 5% CO2 at 37 ºC. Twenty-four hours later, decapeptide-12 or oxyresveratrol was added to the corresponding wells at varying concentrations (0, 3, 10, 30, 100, 300, and 1000 µM), cultures were then incubated for 72 h, and manufacturer’s protocol was followed for the remainder of the procedure.
Trypan blue exclusion assay was used to measure cellular toxicity. Cells were cultured in 6-well plates at a density of 4×105 cells/well and then treated with various concentrations of decapeptide-12 or oxyresveratrol (0, 3, 10, 30,100, 300, and 1000 µM). Plates were incubated at 37ºC in a humidified 5% CO2 chamber. After 72 h, an aliquot was taken, and cells counted using a hemacytometer. Cytotoxicity was measured according to the following formula: [1 − (# of cells in control − # of live cells in test sample)/# of cells in control] × 100%.
NK cytotoxic killing and PBMC proliferation assays
Cryopreserved human peripheral blood mononuclear cells (PBMCs) were purchased from Astarte Biologics (Redmond, WA), activated with phytohemagglutinin (PHA), and proliferation assessed according to previously described method [36]. Interleukin (IL)-2 activated human natural killer (NK) cell cytotoxic killing of human K562 cells was assessed using a CytoTox96 non-radioactive cytotoxicity assay kit (Promega) as previously described [36]. Protease inhibitor was added to the media to prevent degradation of decapeptide-12.
Statistical analysis
Three independent trials were performed for each experiment. Microsoft Excel (Seattle, Washington) was used to calculate means and standard errors and statistical significance was determined using unpaired analysis of variance or two-tailed student T-test. P values <0.05 were taken to be statistically significant.
Results
Effect of decapeptide-12 on keratinocyte progenitor proliferation and cytotoxicity
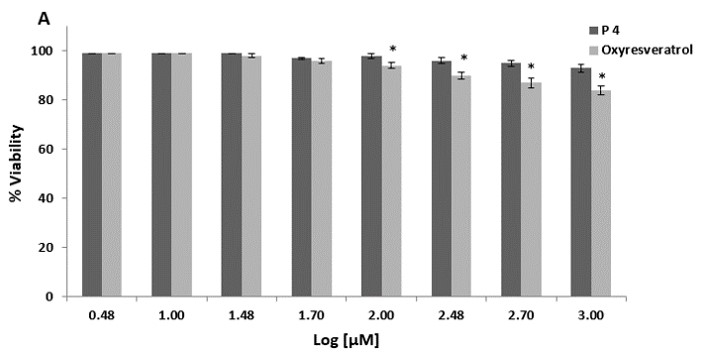
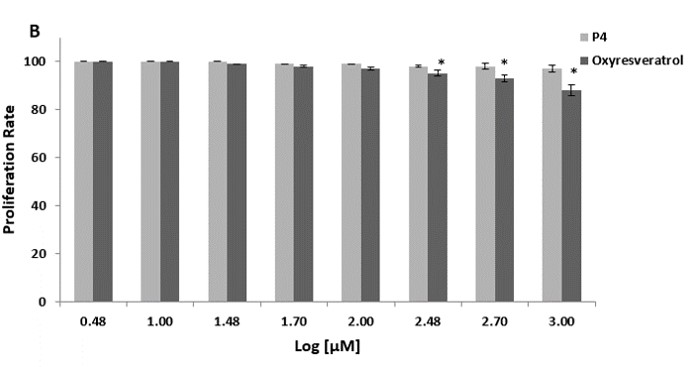
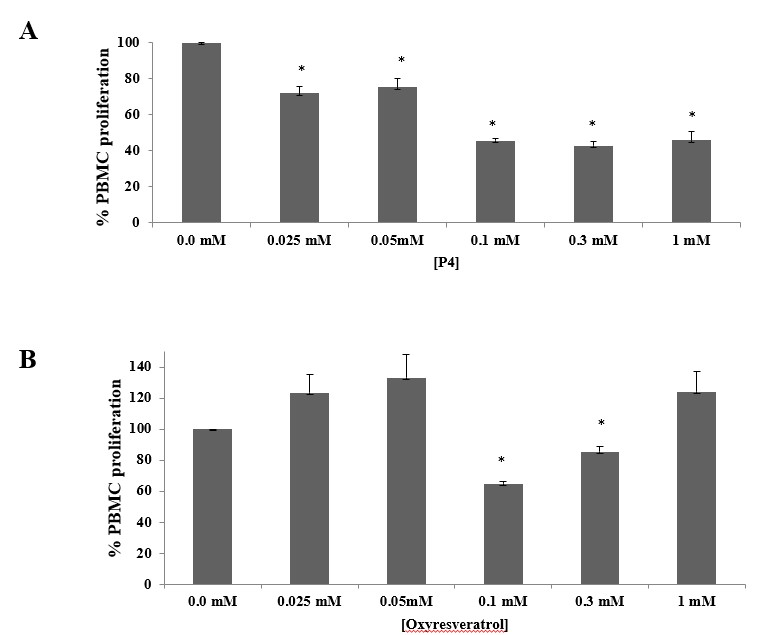
We first assessed the cytotoxic effect of decapeptide-12 and oxyresveratrol on human neonatal keratinocyte progenitors. Treatment with 100 µM of decapeptide-12 and oxyresveratrol resulted in 3 ± 1% and 6 ± 1% cell deaths, respectively (Figure 2a). At 1 mM, decapeptide-12 and oxyresveratrol resulted in 7 ± 2% and 16 ± 2% of cell death, respectively.
We also evaluated the effects of decapeptide-12 and oxyresveratrol on the viability and proliferation of human neonatal keratinocyte progenitors. Figure 2b shows that treatment with 300 µM of decapeptide-12 and oxyresveratrol resulted in 2 ± 1%, and 5 ± 1%, reduction in cell proliferation rates, respectively. However, unlike 1 mM decapeptide-12 which reduced proliferation 3 ± 2%, 3-day incubation with oxyresveratrol reduced proliferation 12 ± 2%.
|
Figure 2: (A) Cytotoxic effects of decapeptide-12 (P4) and oxyresveratrol on human neonatal keratinocyte progenitors. (B) Effects of decapeptide-12 and oxyresveratrol on human neonatal keratinocyte progenitor proliferation. Data are expressed as % control and represent means ± SEM of 3 separate experiments. *P<0.05. |
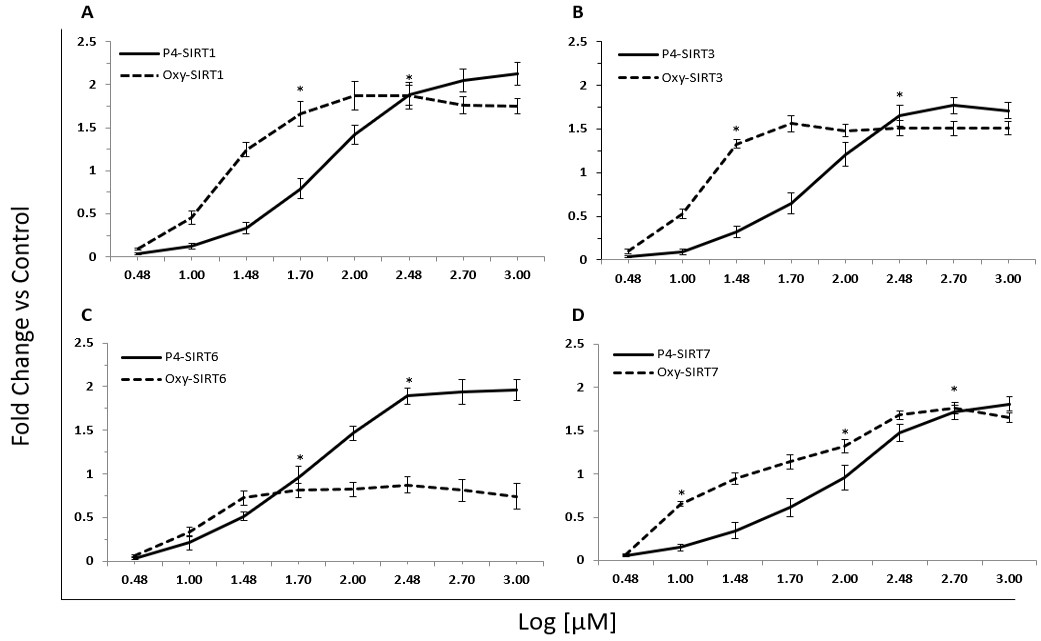
Decapeptide-12 upregulated transcription of SIRT1-7 in keratinocyte progenitors
We next assessed the effect of oxyresveratrol and decapeptide-12 on sirtuin expression in human neonatal keratinocyte progenitors. Figure 3 and table 2 show that treatment with decapeptide-12 and oxyresveratrol modulated transcription of SIRT1-7 in a dose-dependent fashion. At 30 µM of oxyresveratrol, SIRT1 transcription levels were upregulated by 125 ±9% relative to control cells, whereas SIRT1, SIRT6, and SIRT7 were upregulated by 133 ± 5%, 73 ± 8% and 95 ± 7%, respectively. One hundred µM of decapeptide-12 increased transcription of SIRT3 by 141 ± 11% relative to untreated cells, whereas SIRT1, SIRT6 and SIRT7 increased by 121 ± 13%, 147 ± 8% and 95 ± 14%, respectively (Figure 3).
|
Figure 3. Dose-dependent transcriptional upregulation of SIRT3 (a), SIRT3, (b), SIRT6 (c), and SIRT7 by decapeptide-12 (P4) and oxyresveratrol (Oxy) in human neonatal keratinocyte progenitors (d). Data are expressed as fold increase relative to the internal control gene 18S, and represent means ± SEM of 3 independent experiments. *P<0.05. |
Table 2a
Table 2b Table 2. Gene expression profile of SIRT 1-7 in response to treatment with decapeptide-12 (a) and oxyresveratrol (b). Results are averages ± SEMs of 3 independent runs. |
Decapeptide-12 is immunosuppressive
We also explored the effects of decapeptide-12 and oxyresveratrol on PBMC proliferation rates after exposure to PHA for 72 h. Figure 4A shows decapeptide-12 statistically significantly (p<0.02) reduced proliferation by 28 ± 3.8% at 0.05 mM and 54 ± 1 at 0.1 mM. No significant additional reduction was achieved at 0.3 or 1 mM (p>0.05). Oxyresveratrol also reduced proliferation by 35 ± 2% (p<0.02) at 0.1 mM although only by 15 ± 3% at 3 mM (p<0.02) (Fig. 4B). Other concentrations tested did not show a statistically significant change (p>0.14) Figure 4B.
|
Figure 4. Immunosuppressive effects of decapeptide-12 (P4) (A) and oxyresveratrol (B) on PHA-stimulated PBMC proliferation. Data are expressed as % control and represent means ± SEM of 3 separate experiments. *P<0.05. E:T denotes effector to target cell ratio. |
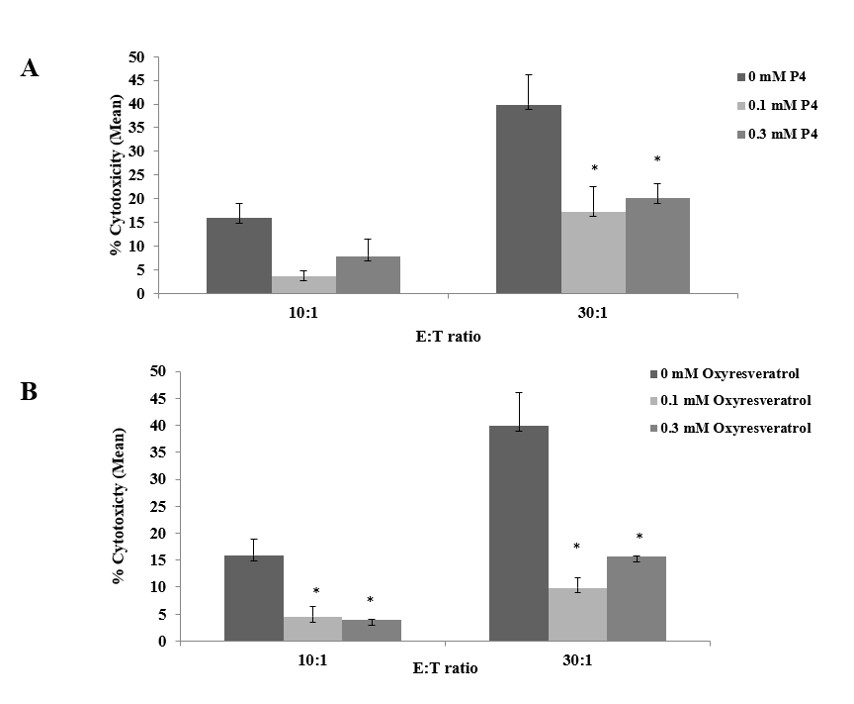
We concluded by assessing the impact of decapeptide-12 on IL-2 primed NK-mediated cytotoxic killing of K562 cells. Figure 5A illustrates that decapeptide-12 reduced NK killing by 81 ± 1% and 59 ± 4% at 0.1 and 0.3 mM, respectively (p>0.05), at 10:1 ratio of effector to target (E:T) cells. At a 30:1 ratio, decapeptide-12 reduced NK killing by 65 ± 5% and 45 ± 3% at 0.1 and 0.3 mM, respectively (p<0.04). Figure 5B shows that oxyresveratrol diminished NK killing by 89 ± 2% and 86.1 ± 0.9% at 0.1 and 0.3 mM, respectively (p<0.03), at E:T ratio of 10:1. At a 30:1 ratio, oxyresveratrol blocked NK killing by 73 ± 2% and 64 ± 3% at 0.1 and 0.3 mM, respectively (p<0.03).
|
Figure 5. Immunosuppressive effects of decapeptide-12 (P4) (A) and oxyresveratrol (B) on NK92-mediated cytotoxic killing of K562 cells. Data are expressed as % control and represent means ± SEM of 3 separate experiments. *P<0.05. E:T denotes effector to target cell ratio. |
Discussion
Sirtuin pleiotropic effects are believed to underlie delayed senescence and aging. Therapeutic use of resveratrol as a SIRT1 activator and potential anti-aging agent has been extensively researched and documented [37-39]. By activating SIRT1, a nuclear deacetylase [26], resveratrol conveys protection of human endothelium from H2O2-induced oxidative stress and senescence [29]. Similarly, oxyresveratrol is also a potent antioxidant and free radical scavenger. However, unlike resveratrol, it exhibits less cytotoxicity and better water solubility. Consequently, we elected to use it as a positive control against which we compared decapeptide-12’s performance and ability to modulate sirtuin transcription in human neonatal keratinocyte progenitors.
Even though all 7 sirtuins were upregulated after treatment with decapeptide-12, our discussion will focus on those sirtuins directly implicated in skin aging. At 100 μM and 1 mM, decapeptide-12 increased SIRT1 transcription by an impressive 141% and 213%, respectively. SIRT1 exerts effects on metabolism, stress response, genome stability, and cell survival, proliferation, and differentiation [40-42]. In keratinocytes, SIRT1 was reported to protect against UVB- and H2O2-induced cell death by modulating p53 and c-Jun N-terminal kinases leading authors to speculate that SIRT1 activators may serve as anti-aging agents [12]. Furthermore, via suppression of NF-κB signaling, SIRT1 may delay aging and extend lifespan [43]. This is achieved by deacetylating the p65 subunit of NF-κB complex leading to improved oxidative metabolism and earlier resolution of inflammation [44]. Thus, SIRT1 has been firmly established as a crucial anti-aging protein [45] .
SIRT3 transcription was increased by 121% following treatment with 100 µM decapeptide. SIRT3 effects include regulation of mitochondrial β-oxidation, ATP generation, ROS [46], and hematopoietic stem cell self-renewal [47]. This latter discovery helps lay the path for future stem cell-based interventions for metabolic disorders resulting in premature aging.
SIRT6 is another sirtuin family member with pleiotropic effects on metabolism, inflammation, tumor suppression, and DNA repair [45]. In a landmark study, SIRT6 knockout caused severe premature aging phenotypes and mouse lifespan reduction to 1 month [48]. Whole body SIRT6 overexpression resulted in increased lifespan in mice [49]. SIRT6 also exerts an anti-inflammatory effect by histone deacetylation of NF-κB target gene promoters thus reducing NF-κB activity [50].
Baohua et al. showed that SIRT6 plays a key role in the process of skin aging via modulation of collagen metabolism and NF-κB signaling. They reported that blocking SIRT6 significantly decreased hydroxyproline content by inhibiting transcription of type 1 collagen, prompting matrix metalloproteinase 1 secretion and increased NF-κB signaling [51]. Hence, decapeptide-12, which significantly enhanced SIRT6 transcription may hold great promise as a therapeutic anti-aging candidate to address the often-concurrent phenotypes of premature skin aging and photodamaged skin.
Our studies also showed that decapeptide-12 and oxyresveratrol exhibited anti-inflammatory effects as measured by two methods: 1) blockade of PHA-stimulated PBMC proliferation, and 2) inhibition of NK-mediated cytotoxic killing. The effect of decapeptide-12 appeared dose-dependent in contrast to oxyresveratrol which exhibited a very narrow inhibitory concentration range. In fact, the general trend, although not statistically significant, showed oxyresveratrol may have biphasic effects as concentrations of 0.3 and 1 mM showed progressively less inhibition than did 0.1 mM. This was not the case for decapeptide-12 which exhibited a plateau or maximum inhibition at 0.1 mM in the concentration range tested. This can be explained by dose-dependent differences in activation of downstream signaling pathways or feedback loops. Indeed, careful examination of dose-dependency curves of sirtuin expression patterns presented herein reveal biphasic effects with higher concentrations becoming inhibitory.
In contrast, abrogation of NK killing appeared dose-dependent for both decapeptide-12 and oxyresveratrol, with the latter showing more pronounced inhibition at all concentrations tested. The inhibitory effects were greater at an E:T ratio of 10:1 than 30:1 for both decapeptide-12 and oxyresveratrol. This may be due to blockade of NKG2D and perforin mediated cytotoxicity but remains to be determined. Our findings are consistent with those of Boscolo et al., who reported that resveratrol strongly inhibits PHA- induced proliferation at 0.1 mM [52]. They suggested that this suppression effect may be due to inhibition of NF-kappa B, which as noted above, is also regulated by sirtuins and strongly linked to immune and inflammatory responses as well as regulation of cell proliferation and apoptosis, amongst other effects.
Taken together, the immunosuppressive effects observed in our study suggests that unique and specific regulatory pathways are engaged in different arms of the immune system. Further studies are needed to clarify these pleiotropic effects. For example, assessment of the impact of these two agents on pro-inflammatory mediators such as TNFα, IL-1β, IFNγ, and IL-6 would be insightful. In addition, determination of translational and other transcriptional effects of activated versus resting PBMCs would be insightful.
In summary, decapeptide-12 was shown in this report to significantly upregulate transcription levels of SIRT3, SIRT1, and SIRT6, a ll 3 of which play significant roles in counteracting skin aging and other age-associated pathologies. Finally, clinical studies with various topical formulations containing decapeptide-12 and/or oxyresveratrol are currently being designed to help validate the in vitro findings and test the efficacy of these potent sirtuin activators in vivo.
Acknowledgements
We are grateful to Takele Teklemariam and Lebiki Bonkaw for their technical assistance in carrying out PBMC and NK assays and respective data analysis.
References
-
Roth M, Chen WY (2014) Sorting out functions of sirtuins in cancer. Oncogene, 33: 1609-1620.
-
Villalba JM, Alcain FJ (2012) Sirtuin activators and inhibitors. Biofactors, 38: 349-359.
Reference annotation:
**29. Resveratrol protects from oxidative stress and senescence via SIRT3 activation.
**30. Resveratrol’s activation of SIRT3 is correlated to effects on age-related diseases.
**31. Resveratrol prevents oxidative stress-induced senescence cultured human keratinocytes.
**32. Our first report on “decapeptide-12” and its potent tyrosinase inhibition.
**36. Discusses modulation of sirtuins as a promising approach to fight aging and senescence.
**37. A comprehensive review of sirtuins; their activators and inhibitors.
 LOGIN
LOGIN REGISTER
REGISTER.png)