Research Article
Post-Whole Brain Radiation Therapy Systemic Treatment Increases Survival
Junliang Liu*
Department of Radiation Oncology, Cancer Care Manitoba, University of Manitoba, Winnipeg, MB, Canada
*Corresponding author: Junliang Liu, Department of Radiation Oncology, Cancer Care Manitoba, Winnipeg, MB, Canada, Tel: +1 2047871927; Fax: +1 2047860194; E-mail: junliang.liu@cancercare.mb.ca
Citation: Liu J (2018) Post-Whole Brain Radiation Therapy Systemic Treatment Increases Survival. Curr Adv Oncol Res Ther 2018: 1-5. doi:https://doi.org/10.29199/CAON.101011
Received Date: 12 October, 2017; Accepted Date: 11 January, 2018; Published Date: 26 January, 2018
Abstract
Purpose/Objective: Brain metastases account for more than one-half of all intracranial malignancies. The prognosis of cancer patients with brain metastases has been dismal even with the availability of surgery, stereotactic radiosurgery, whole brain radiation therapy or the combination of these modalities. This study is to explore the impact of post-whole brain radiotherapy systemic treatment on patient survival.
Material/Methods: From July 1, 2005 to May 1, 2016, a cohort of patients with brain metastases was treated with whole brain radiation therapy, daily treatment, five days per week. Data was analyzed by comparing the survival difference between the group of patients who received post-whole brain radiation therapy systemic treatment and the group of patients who did not have post-whole brain radiation therapy systemic treatment.
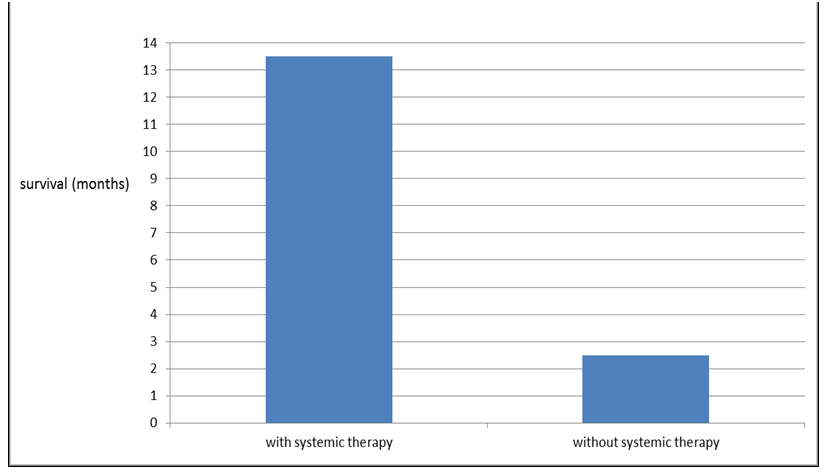
Results: A total of 65 patients, male 31, female 34, aged 25 to 92 years old were identified. Eleven out of 65 patients received post-whole brain radiotherapy systemic treatment including chemotherapy (for 8 out of 11 patients), target therapy (for 2 out of 11 patients), and chemotherapy plus target therapy (for 1 out of 11 patients). The median post-whole brain radiation therapy survival for patients who received post-whole brain radiation therapy systemic treatment was 13.5 months, compared to a much shorter median survival of only 2.5 months for patients who did not receive post-whole brain radiotherapy systemic treatment.
Conclusion: Post-whole brain radiation therapy systemic treatment increases the overall survival of patients with brain metastases.
Keywords: Brain Metastases; Chemotherapy; Systemic Treatment; Target Therapy; Whole Brain Radiation Therapy
Introduction
Brain Metastases (BM) occur in about 10-20% of patients with cancers and account for more than a half of the intra-cranial malignancies [1-3]. Even with the modern advances in surgery, Stereotactic Radiosurgery (SRS), and Whole Brain Radiation Therapy (WBRT), the prognosis of patients with BM is still dismal. With supportive care only, the median survival is about 1 to 2 months, with WBRT, median survival can be increased to 3-6 months [4-6]. Stereotactic radiosurgery is limited by the size, location, and numbers of the metastatic deposits [7,8]. The role of surgery is very much limited to tissue diagnosis in some cases. Most cancer drugs including chemotherapy agents do not cross Blood Brain Barrier (BBB) to be effective [9-11]. The emerging target therapy agents have showed some activities against BM but still with limited duration of responses [12-17]. It is time to explore new ways to treat BM. Based on my personal clinical observation; I noticed that some of the patients survived much longer than the average. With curiosity, I reviewed the literature and I have not found any studies which specifically looked at the impact of post-WBRT systemic therapy on the survival of patients with BM. This retrospective study is to explore the impact of post-WBRT systemic treatment on the survival of patients with BM.
Materials and Methods
From July 1, 2005 to May 1, 2016, a consecutive cohort of patients with brain metastases treated with WBRT was retrospectively studied. All patients were treated with WBRT with daily treatment, five days per week. Most of the patients received either 30 Gy in 10 or 12 fractions. A few patients were treated with 20 Gy in 5 fractions. Patients were referred to medical oncology for post-WBRT systemic therapy. Medical oncologists assessed patients for either chemotherapy or target therapy.
One-year-old patient with breast primary received SRS, 20 Gy to 3 lesions. She was found with multiple new lesions 3 months later and substantial progression of the treated lesions 5.5 months later and re-SRS was not feasible and she was then treated with WBRT.
Aother 48-year-old patient with breast primary and diffuse BM received WBRT initially. Four months later, her BM regrew. She was treated with SRS, 16-18 Gy to 5 lesions. Three months after SRS, her BM had mixed responses, but she developed extensive progression 4 months after SRS.
Statistical Analysis
The survival difference between the group of patients who received post-whole brain radiation therapy systemic treatment and the group of patients who did not have post-whole brain radiation was analyzed statistically by using student t test.
Results
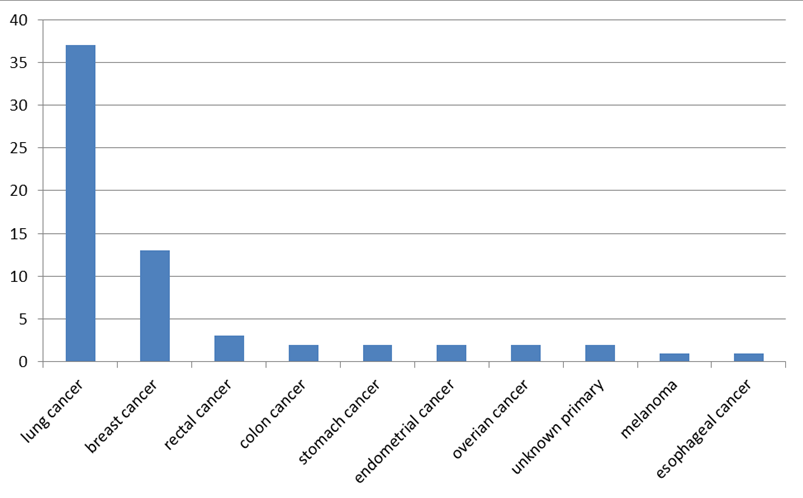
A total of 65 patients, male 31, female 34, aged 25 to 92 years old were identified. Majority of patients were with lung cancer and breast cancer (Table 1, Figure 1). Fifty six out of 65 patients had multiple brain lesions. Sixty two out of 65 patients had extra-cranial diseases. Eleven out of 65 patients received post-WBRT systemic treatment including chemotherapy (8 out of 11 patients), target therapy (2 out of 11 patients), and chemotherapy plus target therapy (1 out of 11 patients) (Table 2). All of those 11 patients had extra-cranial diseases. One out of those eleven patients had a solitary BM (4.8 cm).
|
Figure 1: Patient allocation according to the primary cancer. |
Table 1: Patient Characteristics. |
Table 2: Summary of patients received post-WBRT systemic therapy. |
Patients who received post-WBRT systemic treatment survived longer, compared with those who did not (Figure 2). The median post-WBRT survival for patients who received post-WBRT systemic treatment was 13.5 months, compared to a much short median survival of only 2.5 months for patients who did not receive post-WBRT systemic treatment (P<0.001) (Figure 3). There were only two patients in the non-post-WBRT systemic therapy group survived relatively long with one survived 10 months and another one survived 12 months after WBRT.
|
Figure 2: Post-WBRT percentage month survival. The percentage of survival was calculated by the number of patients who were alive at the end of a 30-day month divided by the total number of patients in each group. |
|
Figure 3: Post-WBRT median survival. |
The observed most common side effects were significant fatigue which started from the second week of WBRT and lasted about 2-3 months, and alopecia which started about 2-3 weeks after radiotherapy and then grew back gradually about 3 months later. Mild short memory decease was observed in some patients.
Impaired hearing ability was noticed for one patient who had some unilateral hearing impairment before treatment and became worse after radiotherapy. This 73 years old man presented with Stage IV lung cancer with spinal and brain metastases. He has been on afatinib after WBRT and radiotherapy to the spinal metastases and he has no visible cranial disease 18 months after WBRT.
The causes of death are very much different between the two groups of patients (Table 3). At least 80.2% of patients who did not receive post-WBRT systemic treatment died of intracranial cancer progression quickly, while 80.0% of patients who received post-WBRT died of extra-cranial cancer progression much later.
Table 3: The causes of death of patients with brain metastases. *One out of 11patients who received post-WBRT target therapy is still alive 20 months after WBRT ¥Patient died of pneumonia |
||||||||||||||||||||||||||||
Discussion
This study demonstrates post-WBRT systemic therapy, chemotherapy or target therapy significantly increases overall survival in patients with BM with a median survival of 13.5 months, compared with a much shorter median survival of only 2.5 months for those who did not receive post-WBRT systemic treatment. It is noticeable that most of the patients in this cohort did not receive post-WBRT systemic treatment. This was due to a number of factors. Some of the patients died quickly after WBRT due to the progression of BM and therefore did not have chance to be offered systemic treatment while some of the patients were not offered systemic therapy due to the concern of their performance status. Often the post-WBRT fatigue was interpreted as poor performance. Some patients with pre-treatment neurological deficits convalesced gradually after WBRT and their performance statuses before a full recovery were deemed not good enough to receive systemic therapy by medical oncologists. As one can see, most of the patients in this study received post-WBRT systemic therapy very late. This was chiefly due to patients’ slow recovery from their neurological deficits due to BM and side effects such as significant fatigue related to WBRT. Overall, medical oncologists are very reluctant to offer systemic therapy, specially chemotherapy to patients with BM because of the perceived poor prognosis in terms of limited life expectancy due to BM even patient’s extra-cranial disease is under control or there is no evidence of extra-cranial disease. There is no doubt that some of those patients who did not receive post-WBRT systemic therapy might benefited from it should they be offered one. This is especially true based on the data that the WBRT effects like the ones from SRS are generally not durable. Therefore, there is a window opportunity for systemic therapy. The best time for systemic treatment to be effective is before the BM regrows again and before the radiation-disrupted BBB is fully repaired again.
Studies showed that the timing of systemic therapy is critic. Upfront systemic treatment is inferior to upfront WBRT. Patients who were treated with upfront target therapy with erlotinib had less durable intracranial response with a significantly shorter time to intracranial progression, and those patients were more likely to experience intracranial failure as a site of first progression [18]. Another study found that both progression-free and overall survival were inferior in patients treated with upfront erlotinib compared with upfront radiotherapy and the death rate due to intracranial progression was higher among patients who received upfront erlotinib [19]. This may indicate that target therapy agent reaches the cancer cells in the brain tissue more sufficiently after the disruption of the BBB by upfront WBRT or the damages of cancer cells are more efficient or more effective by WBRT initially when the patient is eligible to receive target therapy.
Concomitant systemic treatment with WBRT was tried in several studies. The Radiation Therapy Oncology Group (RTOG) conducted a phase III study, 126 patients with Non-Small Cell Lung Cancer (NSCLC) and 1 to 3 brain metastases were treated with WBRT plus SRS, WBRT plus SRS plus temozolomide, or WBRT plus SRS plus erlotinib [20]. The overall survival was much shorter in each of the combined drug therapy with WBRT arms compared with WBRT/SRS alone, and toxicity was significantly higher in patients who received concomitant drug therapy. The results of this study suggest that concomitant systemic treatment with WBRT is detrimental.
It has been well documented that the BBB is disrupted by brain irradiation and the destruction of the BBB in irradiated brain tissue showed a linear relation with radiation dose. After administering a total dose of 30 Gy the permeability increased to 75% and this returns to pre-treatment levels 8 moths later and the BBB recovered completely in normal brain tissue [21]. This provide a therapeutic window opportunity for systemic agents to reach the parenchyma of the brain, especially for those chemotherapy drugs which do not cross the normal BBB at all or cross with insufficient amount.
Surgery may add survival benefit onto WBRT but this has been only proved in patients with solitary BM [22-24]. The overall survival of these highly selected patients was only about 10 months. Patients with single BM resected by surgery have a 50-60% risk of local recurrence at the surgical site within 6-12 months [8,25,26]. In our study, there were only a small number of patients who had solitary BM but none of those was a candidate for neurosurgery. All, except one, of our patients who received post-WBRT systemic therapy had multiple BMs and yet the median overall survival for this group of patients reached 13.5 months which is apparently superior to that achieved in patients with solitary BM resected by surgery followed by WBRT in the literature
SRS was associated with inferior surgical site control as well as much higher intracranial recurrence and the local recurrence was even higher if the preoperative tumor diameter is larger than 2.5 cm. The adjunctive WBRT reduced the risk of intracranial disease recurrence or progression by about 50% compared with SRS alone, but it has not been shown to extend overall survival [27-29]. Furthermore, the response duration seems as short as WBRT alone and the high rate of intracranial failure associated with SRS often is not salvageable by re-SRS or WBRT as indicated in this study.
The intrinsic sensitivity of a tumor to chemotherapy agent is as critical as the ability of the drug to reach the brain tissue. This has been well demonstrated by studies that combined temozolomide with WBRT. Temozolomide has not been used as a part of the standard chemotherapy to the extra-cranial lung cancer due to its lack of intrinsic sensitivity of lung cancer. Because it can easily cross the BBB and presents in the brain parenchyma, it was used to combine with WBRT in treating patients with BM from NSCLC in several clinical trials without favorable results [30,31].
The arguments in favor of using SRS instead of WBRT in treating patients with BM have been the concern of possible long term neurocognitive decline related to WBRT. However, those were mostly based on limited period of observations. In one multi-institute randomized study, patients with 1 to 3 brain metastases were randomized to receive SRS or SRS plus WBRT. There was less cognitive deterioration at 3 months after SRS alone than when combined with WBRT, but time to intracranial failure was significantly shorter for SRS alone compared with SRS plus WBRT, and there was no significant difference in functional independence at 3 months between the treatment groups. For long-term survivors, the incidence of cognitive deterioration was less after SRS alone at 3 months and but the difference was significantly narrowed at 12 months [29]. A more recent study [32] has found that the overall prevalence of leukoencephalopathy (significant diffuse white matter changes and it usually implies a neurocognitive decline) was 42, 60, 73 and 84% at 1, 2, 3, and 4 years after SRS. This study indicated that long-term BM survivors treated with SRS are at progressive risk for developing leukoencephalopathy which may lead to neurocognitive decline even dementia, especially for those with a higher BM burden, higher integral SRS dose to the brain.
Our current study clearly brings a new and better approach in treating patients with BM. It is especially useful for those patients whose extra-cranial diseases have controlled by effective chemotherapy drugs which are unable to cross the BBB to deal with their BMs. The limitations of this study are retrospective and the number of patients is relatively small. Further study is planned to include the whole institutional cohort of patients. Nevertheless, considering the dismal average survival of patients with BMs who were treated with single modality with WBRT, SRS, surgery or combined surgery plus WBRT or SRS, our study has shed a light on new direction in managing patients with BMs.
Conclusion
The effects of WBRT alone are short. Recurrence at the same sites or new intracranial metastases is very high for patients treated by surgery or SRS alone. Single modality alone rarely induces adequate or durable control of the disease for patients with BM. The effects of systemic therapy including chemotherapy and or target therapy alone is short-lived even the agent can cross the BBB. Systemic therapy should be aggressively considered after upfront brain irradiation. SRS may cause isolated disruption of the BBB at the sites of treatments and this may not be enough for systemic therapeutic agents to reach the brain tissue which harbors microscopic or small metastatic deposits which are not targeted by SRS. The best way to manage BM with better outcome is to pursue multidisciplinary or multimodality approaches such as upfront WBRT followed by systemic therapy either chemotherapy or and target therapy.
References
- Posner JB, Chernik NL (1978) Intracranial metastases from systemic cancer. Adv Neurol 19: 579-592.
- Takakura K, Sano K, Hojo S, Hirano A (1982) Metastatic tumors of the central nervous system. In: Central Nervous System Diseases. 1stedn, Igaku-Shoin, Tokyo, Japan. Pg no: 346.
- International Agency for Research on Cancer (IARC) (2014) In: Stewart BW, Wild CP (eds.). World Cancer Report 2014. World Health Organization (WHO), Geneva, Switzerland.
- Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, et al. (2003) Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21: 2529-2536.
- Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, et al. (2006) Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol 24: 106-114.
- Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, et al. (1980) The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 6: 1-9.
- Hartford AC, Paravati AJ, Spire WJ, Li Z, Jarvis LA, et al. (2013) Postoperative stereotactic radiosurgery without whole-brain radiation therapy for brain metastases: potential role of preoperative tumor size. Int J Radiat Oncol Biol Phys 85: 650-655.
- Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, et al. (2017) Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 18: 1040-1048.
- Zhang RD, Price JE, Fujimaki T, Bucana CD, Fidler IJ (1992) Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol 141: 1115-1124.
- Stewart DJ (1994) A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol 20: 121-139.
- Davey P (2002) Brain metastases: treatment options to improve outcomes. CNS Drugs 16: 325-338.
- Chiu CH, Tsai CM, Chen YM, Chiang SC, Liou JL, et al. (2005) Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 47: 129-138.
- Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crinò L, et al. (2004) Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 15: 1042-1047.
- Wu C, Li YL, Wang ZM, Li Z, Zhang TX, et al. (2007) Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 57: 359-364.
- Kim JE, Lee DH, Choi Y, Yoon DH, Kim SW, et al. (2009) Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 65: 351-354.
- Zee Y-K, Chin T-M, Wong ASC (2009) Fatal cystic change of brain metastasis after response to gefitinib in non-small-cell lung cancer. J Clin Oncol 27: 145-146.
- Porta R, Sánchez-Torres JM, Paz-Ares L, Massutí B, Reguart N, et al. (2011) Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 37: 624-631.
- Gerber NK, Yamada Y, Rimner A, Shi W, Riely GJ, et al. (2014) Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys 89: 322-329.
- Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, et al. (2016) Impact of Deferring Radiation Therapy in Patients with Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Develop Brain Metastases. Int J Radiat Oncol Biol Phys 95: 673-679.
- Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, et al. (2013) A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys 85: 1312-1318.
- Qin DX, Zheng R, Tang J, Li JX, Hu Y (1990) Influence of radiation on the blood-brain barrier and optimum time of chemotherapy Int J Radiat Oncol Biol Phys 19: 1507-1510.
- Roy A, Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, et al. (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322: 494-500.
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, et al. (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33: 583-590.
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, et al. (1994) The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29: 711-717.
- Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, et al. (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280: 1485-1489.
- Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, et al. (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29: 134-141.
- Soon YY, Tham IW, Lim KH, Koh WH, Lu JJ (2014) Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev 2014: CD009454.
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, et al. (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10: 1037-1044.
- Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, et al. (2016) Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 316: 401-409.
- Chua D, Krzakowski M, Chouaid C, Pallotta MG, Martinez JI, et al. (2011) Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: a randomized, open-label phase II study. Clin Lung Cancer 11: 176-181.
- Robins HI, O’Neill A, Mehta M, Grossman S (2013) A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT & SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320: in regard to Sperduto et al. Int J Radiat Oncol Biol Phys 86: 809-810.
- Cohen-Inbar O, Melmer P, Lee CC, Xu Z, Schlesinger D, et al. (2016) Leukoencephalopathy in long term brain metastases survivors treated with radiosurgery. J Neurooncol 126: 289-298.
 LOGIN
LOGIN REGISTER
REGISTER.png)