Research Article
Short-Term Stability Study of Doxycycline Tablets by High Performance Liquid Chromatography, Spectrophotometry in the Ultraviolet Region and Turbidimetry
Janaína Oliveira de Barros, Ana Carolina Kogawa* and Hérida Regina Nunes Salgado
São Paulo State University (UNESP), School of Pharmaceutical Sciences, Campus Araraquara, São Paulo, Brazil
*Corresponding author: Ana Carolina Kogawa, Faculdade de Ciências Farmacêuticas de Araraquara, UNESP, Rodovia Araraquara-Jaú, Km 1, CEP 14800-903, Araraquara, SP, Brazil, Tel: +55 1633014681, +55 163301-6963; Fax: +55 1633016960; E-mail: ac_kogawa@yahoo.com.br
Citation: de Barros JO, Kogawa AC, Salgado HRN (2017) Short-Term Stability Study of Doxycycline Tablets by High Performance Liquid Chromatography, Spectrophotometry in the Ultraviolet Region and Turbidimetry. J Pharmacol Clin Trials 2018: 43-49. doi: https://doi.org/10.29199/JPCT.101018
Received Date: 05 December, 2017; Accepted Date: 10 January, 2018; Published Date: 26 January, 2018
Abstract
Doxycycline, an antimicrobial, is available for free in the Brazilian Public System under medical prescription. Therefore, the population have easy access to it. Regarding quality control, doxycycline stability studies are important due to the epimerization reactions and the formation of degradation products under conditions that are not favourable to the drug leading to severe damages to the patient. So, the aim of this study was to develop a short-term stability study of doxycycline hyclate in intact tablet, pulverized tablets and in raw material. It utilized spectrophotometry in the Ultraviolet Region (UV), High Performance Liquid Chromatography (HPLC) and microbiologic method of turbidimetry. The combination of physicochemical and microbiological techniques is important in the sense that it is not enough only to identify and quantify the drug, but also to verify the activity. The short-term stability study proved that doxycycline hyclate in raw material, intact tablet and pulverized tablets was unstable under extreme conditions of heat and humidity 40ºC ± 2.0ºC and 75% ± 5.0%. The results were significantly different at time 0 and time 6. Raw material followed order zero degradation equations for spectrophotometry and HPLC and second order equation for turbidimetry. Intact tablet and pulverized tablets followed order zero equations for HPLC and second order equation for spectrophotometry and turbidimetry. There was a decrease in the antimicrobial activity and possible formation of degradation products that were detected at the final time of the study. The method can also be used in the test routine of pharmaceutical quality control for the efficacy and reliability of doxycycline tablets.
Keywords: Doxycycline Hyclate; High Performance Liquid Chromatography (HPLC); Short-Term Stability Study; Spectrophotometry in the Ultraviolet Region; Tablets; Turbidimetry
Introduction
Tetracyclines were first discovered around 1945 and were known to be produced from species of Streptomyces. In general, tetracyclines are bacteriostatic agents and out of several advantages, the broad-spectrum activity and low toxicity are the most important compared to other classes of antibiotics. The variability in the structures of this class of drug is responsible for the broad-spectrum activity, that is, this variability allows several interactions between the drugs and different molecules of the body [1,2].
This class of antibiotics is considered as a bacteriostatic agent because of its mechanism of action. Tetracyclines link to the bacteria’s 30S ribosome and in this way the transcription of the genetic information is blocked. In a consequence, the production of bacterial proteins does not occur [3].
Tetracyclines are divided into three generations: natural, semisynthetic of second and of third generation [1]. Within the semisynthetic tetracyclines of second generation, there is Doxycycline (DOX). It is one of the most important compounds because it presents a good lipophilicity which makes it possible for DOX to permeate into the cell membranes more easily [2].
DOX is produced from oxytetracycline and during the process an intermediate product is formed: methacycline. Also, during the process, 6-epidoxycycline can be formed as a residual product. As being a tetracycline, DOX presents a broad-spectrum activity and it acts against Gram-positive and Gram-negative bacteria [4,5].
In the framework of Brazilian Public System called “Sistema Único de Saúde” (SUS), DOX is in the National Relation of Medicines called RENAME [6] and thereby it is available for free at the Health Basic Units under medical prescription. DOX is available in RENAME in tablets of 100 mg and powder for injectable solution of 100 mg.
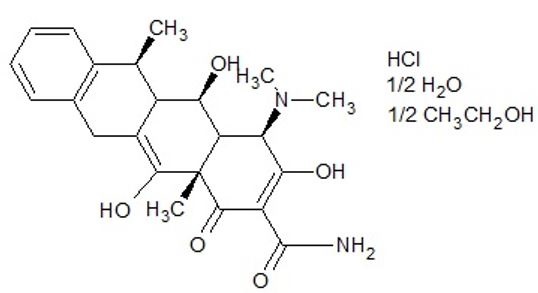
Doxycycline hyclate (Figure 1) is chemically named 4- (dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamideand IUPAC name of Hydrochloride Hemiethanol Hemihydrate of Doxycycline (DOXH). It is shown as a yellow, crystalline and hygroscopic powder. It has a good solubility in water and in methanol and a moderate solubility in ethanol. The content limits are in the range of 95% - 102% [7].
|
Figure 1: Chemical structure of doxycycline hyclate. |
In general, tetracyclines can suffer epimerization under unfavourable conditions of temperature, pH and humidity. In relation to DOX, this reaction can occur at C-4 and C-6 positions resulting in degradation products [5]. These degradation products are: 4-epidoxycycline (4-EDOX), 4,6-epidoxycycline (4,6- EDOX) and 2-acetyl-2-decarboxiamidodoxycycline (ADDOX) [8]. These degradation products reduce antimicrobial activity and can lead to severe hepatotoxicity [9].
The limits for each degradation product were established by the United States Pharmacopoeia [10], European Pharmacopoeia [11] and International Pharmacopoeia and they are: 0.5% for 4-EDOX, 0.5% for 4,6- EDOX and 0.5% for ADDOX.
Thus, DOX stability studies are important due to the epimerization reactions and the formation of degradation products under conditions that are not favourable to the drug leading to severe damages to the patient. Therefore, the quality control is essential to maintain the patient’s security.
According to the literature, physicochemical methods are the most utilized in vivo and in vitro identification and quantification of DOX, within then, High Performance Liquid Chromatography (HPLC) has been considered the main one [3,4] and [12-14]. However, methods like capillary electrophoresis and spectrophotometry are present in those analyses as well and have the advantage of being more easily to handle [8] and [15-17].
According to World Health Organization (WHO), the stability of a drug is defined as its capability of maintaining its physicochemical, microbiologic and biopharmaceutical properties within its expiration time [18]. Also, it is considered the period that the drug keeps its characteristics from the moment of its fabrication until its use by the patient [19].
This stability depends on several environmental factors, such as temperature, humidity and light. Furthermore, the drug’s intrinsic factors also have influence on its stability, and these factors are related to the pharmaceutical formulae, fabrication process and packing materials [20,21].
The stability is important for the pre-registration stage of the drug and it is required by the Brazilian regulatory agency - ANVISA. In that sense, short and long-term stability studies present effective methods to analyse and guarantee the quality of the drugs and therefore they play an important role for the pharmaceutical industries regarding quality control [19,22,23].
The short-term stability study aims to speed up the chemical or physical degradation of the drugs [24,25]. This accelerated degradation is important because it makes it possible to identify degradation products that can be formed during bad stock or transportation conditions [21].
The aim of this study was to develop a short-term stability study of doxycycline hyclate in intact tablet, pulverized tablets and in raw material; it utilized spectrophotometry in the Ultraviolet Region, High Performance Liquid Chromatography and microbiologic method of turbidimetry.
Experimental Part
Materials
The raw material was doxycycline hyclate provided by UniãoQuímicaTM with a content of 97.0%. The sample used in this study was doxycycline hyclate 80 mg - DoxitratTM.
Solutions and reagents: The spectrophotometric method used hydrochloric acid 0.01 mol/L for the preparation of solutions containing the raw material and tablets; purified water was used to set the equipment’s baseline.
The HPLC method utilized a mixture of deionized water Milli Q + 0.1% Trifluoroacetic Acid (TFA): Acetonitrile (CAN) + 0.1% mol/L and acetonitrile with analytical grade as a mobile phase [26].
The microbiological method using turbidimetry utilized Escherichia coli strain ATCC 10536 cultivated and kept in tryptic soy agar and replicated to Brain Heart Infusion medium (OxoidTM). The samples preparation utilized purified water. Formaldehyde 12% analytical grade (QuemisTM) was used to stop the growth of the microorganisms.
Equipment: The raw material and the samples were weighted on analytical scale model H51 (Mettler ToledoTM). All of the other products were weighted on semi-analytical scale model BG 1000 (GehakaTM).
Samples were maintained into climatic chamber MA 835/UR brand MarconiTM at 40ºC and 75% humidity.
Spectrophotometry used UV/Vis spectrophotometer ShimadzuTM model UV mini-1240, cell with length path of 1 cm at wavelength of 268 nm.
HPLC was carried out on Waters system, model 1525 brand WatersTM Chromatography Systems, connected to a 360-wavelegnth-UV/Visible detector WatersTM 2487 and manual injector 7725i with loop of 20 µL brand Rheodyne BreezeTM. The drug analysis was conducted in Phenomenex Luna CN C15 (5 µ 250 x 4.6 nm) column with a flow rate of 1.0 mL/min and temperature kept at 25ºC ± 1ºC.
For the microbiological assay, test tubes were sterilized in vertical autoclave AV brand Phoenix LufercoTM was used for the material’s sterilization. The incubation used Shaker model MA420 brand MarconiTM and hothouse ECB 1.2 digital OdontobrásTM. Results were analysed using spectrophotometer model DU530 brand Beckman CoulterTM.
Methods
Determination of average weight
The average mass is important for the quality control of drugs. A total of 20 tablets were weighted in order to determine the average weight. Doxycycline hyclate lies under the category of “uncoated tablets” and according to the Brazilian Pharmacopoeia [7], 2 out of 20 units can be outside the variation limit.
Sample preparation
In order to obtain the pulverized tablets, 10 of 20 tablets previously weighted were pulverized in porcelain mortar. The other 10 tablets were kept intact into climatic chamber and only one tablet was pulverized in the moment of the analysis to prepare the main solution. Raw material did not suffer any treatment. All the samples were kept in glass discs.
Stability study
This study analysed doxycycline hyclate in extreme conditions of temperature and humidity (40ºC and 75% humidity) and it used methods that were previously developed and validated [26-28]. Samples were analysed at three different periods: 0, 3 and 6 months using the methods already mentioned.
UV/Visible spectrophotometry
The main solution of 100 µg/mL contained 0.04608 g of intact tablet, 0.04608 g of pulverized tablets and 0.01 g of the raw material. They were prepared in amber volumetric flasks with hydrochloric acid 0.01 mol/L. An aliquot of 3.75 mL was removed from each flask and transferred to a 25-volumetric flask to result in a final concentration of 15 µg/mL. The samples were read at wavelength of 268 nm.
High Performance Liquid Chromatography
The preparation of the main solutions followed the same steps as the spectrophotometry. An aliquot of 20 µL was removed from the main solutions (100 µg/mL) and transferred to a 25 mL volumetric flask in order to obtain a final solution of 80 µg/mL. The samples were read at wavelength of 360 nm.
Microbiologic analysis - Turbidimetric assay
The preparation of the main solutions followed the same steps as the spectrophotometry. An aliquot of 1.5 mL was removed from each flask and transferred to a 25 mL volumetric flask resulting in a final concentration of 6 µg/mL.
For each sample, 10 test tubes were prepared containing 10 mL of broth Brain Heart Infusion (BHI) and 2 tubes were used for the negative and positive control. All the samples were sterilized. Each tube containing 10 mL of BHI, received 200 µL of the samples followed by 1 mL of the inoculum. All the tubes were incubated into the Shaker for 4 hours at 35.0ºC and rpm= 25. At the end of the incubation, 500 µL of formaldehyde 12% was added to stop the bacteria growth. The samples were read at 530 nm.
Results
Determination of average weight
The average mass of doxycycline tablets was 0.368605g with RSD (%) = 2.37. No unit was outside the variation of ± 10% from the average mass which followed the requirement of the Brazilian Pharmacopoeia [7].
Stability study
The results of the stability study were analysed according to the chemical degradation kinetics. The chemical kinetics refers to the velocity of the reaction that is influenced by many factors. This velocity is represented by a constant k which indicates the intensity of degradation or alteration of drug’s compounds during a period [29].
There are three types of chemical kinetics reactions, order zero, first order and second order. The order of the reaction is experimentally determined by the regression coefficient. The coefficient (r) that is closer to 1 indicates the order of the reaction [30-32].
The zero - order and second - order models were used to describe the degradation kinetics of doxycycline hyclate samples used in this study. The doxycycline content at time zero was calculated using previously validated methods of UV, HPLC and turbidimetry [26-28].
Raw material
Table 1 shows the results of content and linear regression coefficients for doxycycline hyclate in raw material obtained by the methods at time 0, 3 and 6 months under thermal degradation in climatic chamber (40ºC / 75%).
Table 1: Linear regression coefficients of doxycycline hyclate - raw material - at 0, 3 and 6 months determined by spectrophotometry UV/Vis, HPLC and turbidimetry. |
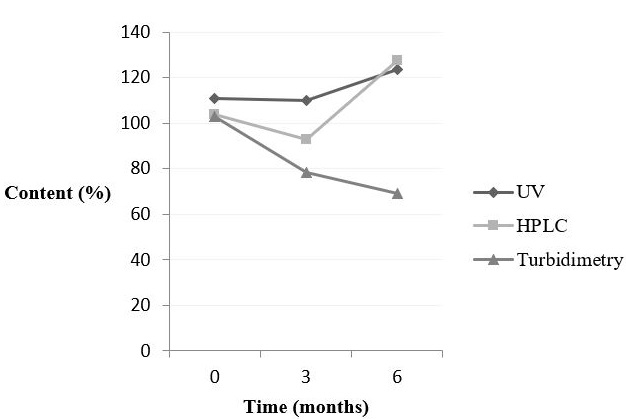
Whereas, figure 2 shows the behaviour of doxycycline hyclate in raw material for each method and time.
|
Figure 2: Behaviour of doxycycline hyclate - raw material - analysed by spectrophotometry UV/Vis, HPLC and turbidimetry. |
Spectrophotometry and HPCL presented a decrease at time 3 followed by an increase in content at time 6. This may be due to co-elution of degradation products formed during the 6-month stability study. These possible degradation products should not present microbiological activity, as the turbidimetric analysis showed a decrease in the potency of doxycycline after 6 months. The statistical analysis showed that the results at time 6 are significantly different from the initial time for all the methods.
Intact tablet: Table 2 shows the results of content and linear regression coefficients for doxycycline hyclate in intact tablet obtained by the methods at time 0, 3 and 6 months under thermal degradation in climatic chamber (40ºC / 75%).
Table 2: Linear regression coefficients of doxycycline hyclate - intact tablet - at 0, 3 and 6 months determined by spectrophotometry UV/Vis, HPLC and turbidimetry. |
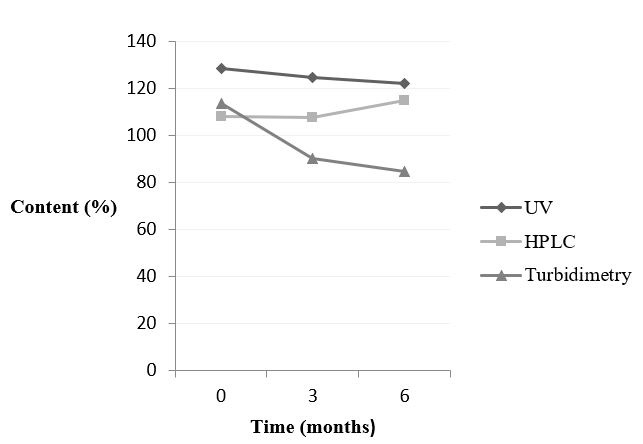
Whereas, figure 3 shows the behaviour of doxycycline hyclate in intact tablet for each method and time.
|
Figure 3: Behaviour of doxycycline hyclate - intact tablets- analysed by spectrophotometry UV/Vis, HPLC and turbidimetry. |
Pulverized tablets: Table 3 shows the results of content and linear regression coefficients for doxycycline hyclate in pulverized tablets obtained by the methods at time 0, 3 and 6 months under thermal degradation in climatic chamber (40ºC / 75%).
Table 3: Linear regression coefficients of doxycycline hyclate - pulverized tablets - at 0, 3 and 6 months determined by spectrophotometry UV/Vis, HPLC and turbidimetry. |
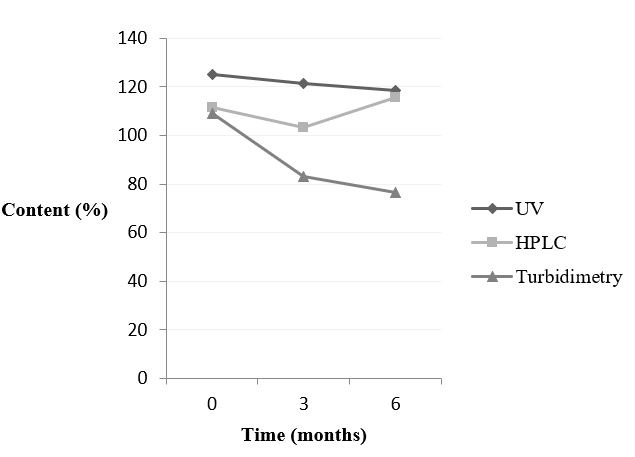
Whereas, figure 4 shows the behaviour of doxycycline hyclate in intact tablet for each method and time.
|
Figure 4: Behaviour of doxycycline hyclate - pulverized tablets - analysed by spectrophotometry UV/Vis, HPLC and turbidimetry. |
The samples of doxycycline hyclate in intact tablet and pulverized tablets showed similar results. The increase in content was detected only by HPLC at time 6. Yet, the content decreased when the samples were analysed by spectrophotometry UV/Vis and turbidimetry during the period of the study. The statistical analysis compared time 0 and 6 and it confirmed that the values were significantly different.
Statistical analysis
The average of the values obtained from the methods at time 0 and 6 were statistically analysed and the results are shown in tables 4-6.
Table 4: Calculated values using F and T tests for the variation within the obtained results for the stability of doxycycline hyclate - raw material - at time 0 and 6. |
Table 5: Calculated values using F and T tests for the variation within the obtained results for the stability of doxycycline hyclate - intact tablet - at time 0 and 6. |
Table 6: Calculated values using F and T tests for the variation within the obtained results for the stability of doxycycline hyclate - pulverized tablets - at time 0 and 6. |
Discussion
Stability studies can be complex because there are many factors that may influence the degradation of a drug, for example: pharmaceutical form, fabrication process, heat, humidity, storage etc. In a consequence, a range of degradation reactions can occur such as oxidation, hydrolysis and epimerization. Such reactions result in drug’s modifications and in case of antibiotics it can decrease its microbiological activity, as well as formation of degradation products [21].
In this sense, tetracyclines are not stable under stress conditions so the epimerization can occur. Being part of the family of tetracyclines, DOX might also suffer this reaction resulting in a change of its properties [5].
This study developed a short-term stability study in doxycycline hyclate samples and the results followed degradation kinetics reactions of order zero and second order. In order zero reactions the velocity of the drug’s degradation is independent of its concentration. On the other hand, for second order reactions, the velocity does depend on the concentration of the drug [29]. As a result, the velocity of degradation of doxycycline hyclate is influenced by heat and humidity.
Raw material
This sample followed order zero degradation equations for spectrophotometry and HPLC and second order equation for turbidimetry.
Analysing the results by UV and HPLC, the content of DOXH in raw material increased significantly at time 6. The degradation products may have been absorbed at the same wavelength as DOXH both for UV and HPLC due to the similarity within their structures, what can explain this increase. Besides, HPLC was not possible to separate the products from DOXH resulting in co-elution.
The microbiological assay showed that despite the increase of content detected by the previous methods, there was a significant loss of activity at time 6 which dropped to 69.17% in relation to the initial time. This behaviour was already expected since the short-term stability study results in a decrease of efficiency of the drug due to the damage of its active ingredient that is caused by degradation.
Thus, according to the tests, DOXH in raw material was unstable under extreme conditions of heat and humidity after a 6-month time of storage in climatic chamber, resulting in degradation products and loss of microbiological activity.
Intact tablet and pulverized tablets
Both samples followed order zero equations for HPLC and second order equation for spectrophotometry and turbidimetry.
They were grouped together due to the similar results regarding their behaviours under the same exposition to stress factors. The fact that they are finished products can explain why they were different from the raw material.
The spectrophotometry showed that both samples presented a decrease in their content from time 0 to time 6. This decrease was statistically different when time 0 and 6 were compared. A possible explanation is that the degradation products could not be detected by the wavelength set for the experiment (268 nm) and this could be influenced by the presence of the tablet’s excipient.
Nevertheless, when the samples were analysed by HPLC the results were similar to the ones found for the raw material, which makes it a more precise method. The content of intact tablet and pulverized tablets increased significantly at the final time, however this increase was lower than what it was seen for raw material. The degradation products can also explain this situation.
In consonance with the results obtained by turbidimetry for raw material, the content of intact tablet and pulverized tablets also showed a decrease, indicating loss of activity. The decrease in content for the tablets was lower than in the raw material because the tablets can still count on the protection of their excipient which are not present in raw material.
Therefore, both intact tablet and pulverized tablets were unstable under stress conditions of heat and humidity after a 6-month period, presenting a drop of anti-microbiological activity and formation of degradation products.
Although the results of intact tablet and pulverized tablets were similar, when statistically compared they were considered different for spectrophotometry and turbidimetry. In this sense, the content of pulverized tablets was inferior to the content of intact tablet. This may happen due to the overkill process that the pulverized tablets go through. The pulverization is a stressing procedure and it can lead to a false stability of the drug. Thus, it is recommended to carry out a stability study only with intact tablets.
The objective of this study was to evaluate the stability of doxycycline hyclate during a 6-month period under stress conditions. The changes were significant at the final time of the study and this corroborates with results of Rade and collaborators [5] that also developed a thermal degradation of doxycycline hyclate.
These results demonstrate the importance of stability studies during the fabrication and registration process of drugs and medicines, in order to keep with the quality and safety. Besides, this study also revealed that physicochemical methods are useful and precise for the quality control, but the combination with microbiological tests is indispensable when it comes to obtain even more secure results, since they present the activity of the drug [33].
Conclusion
The raw material and tablets showed different results when analysed by spectrophotometry and HPLC, possibly due to the presence of the excipient in finished products. HPLC and spectrophotometry could detect a possible formation of DOXH degradation products at time 6 months.
All the samples presented a decrease of content when analysed by turbidimetry, showing a loss of activity. When the results were statically compared, they were significantly different at time 0 and time 6 for all the methods. Therefore, doxycycline hyclate in raw material, intact tablet and pulverized tablets was sensible under exposition to 40ºC of temperature and 75% of humidity.
For quality control of drugs and medicines, the combination of physicochemical and microbiological techniques is important in the sense that it is not enough only to identify and quantify the drug, but also to verify the activity.
Acknowledgement
The authors acknowledge CNPq (Brasília, Brazil), FAPESP (São Paulo, Brazil) and CAPES (São Paulo, Brazil).
References
- Zakeri B, Wright GD (2008) Chemical biology of tetracycline antibiotics. Biochemistry Cell Biology 86: 124-136.
- Pereira-Maia EC, Silva PP, Almeida WB, Santos HF, Marcial BL, et al. (2010) TETRACICLINAS E GLICILCICLINAS: UMA VISÃO GERAL. Química Nova 33: 700-706.
- Romeu GA, Kano EK, Rolim CMB, Serra CHR, Ferraz HG, et al. (2007) Desenvolvimento e validação de método analítico para quantificação de doxiciclina em plasma humano. Revista Brasileira em Promoção da Saúde 20: 193-198.
- Cinquina AL, Longo F, Anastasi G, Gianneti L, Cozzani R (2003) Validation of a high-performance liquid chromatography method for the determination of oxytetracycline, tetracycline, chlortetracycline and doxycycline in bovine milk and muscle. Journal of Chromatography A 987: 227-233.
- Rade I, Vukosava MD, Branislava S (2007) Thermostability Testing and Degradation Profiles of Doxycycline in Bulk, Tablets, and Capsules by HPLC. Journal of Chromatographic Science 45: 623-628.
- Ministério da Saúde (2015) Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Assistência Farmacêutica e Insumos Estratégicos, Relação Nacional de Medicamentos Essenciais, Rename 2014. 9thedn, Brasília, Brazil.
- Farmacopeia Brasileira (2010) Agência Nacional de Vigilância Sanitária, Farmacopeia Brasileira. 5thedn, Atheneu, São Paulo, Brazil.
- Van Schepdael A, Kibaya R, Roets E, Hoogmartens J (1995) Analysis of doxycycline by capillary electrophoresis. Chromatographia 41: 367-369.
- Yamamoto CH (1999) Aspectos da qualidade da tetraciclina em preparações farmacêuticas sólidas. Correlação entre os métodos de dosagem por cromatografia líquida de alta eficiência e turbidimétrico. Tese, Doutorado em Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, Brazil.
- US Pharmacopeia (2016) National Formulary. 39thedn, United States Convention, Rockville, USA.
- Council of Europe. European Directorate for the Quality of Medicines & HealthCare (EDQM) (2011) European pharmacopoeia. 7thedn, Council of Europe, Strasbourg, France.
- Santos MDF, Vemeersch H, Remon JP, Schelkens M, De Backer P, et al. (1996) Validation of a high-performance liquid chromatographic method for the determination of doxycycline in turkey plasma. Journal of Chromatography B 682: 301-308.
- Yasin A, Jefferies TM (1988) Analysis of tetracycline antibiotics and their common impurities by high-performance liquid chromatography using a polymeric column. Journal of Pharmaceutical and Biomedical Analysis 6: 867-873.
- Denobile M, Nascimento ES (2004) Method validation for the determination of the antibiotics residues oxytetracycline, tetracycline, chlortetracycline and doxycycline in milk by high performance liquid chromatography. Rev Bras Cienc Farm 40: 209-218.
- Aguiar G, Faria LG, Ferraz HG, Serra CHR, Porta V (2005) In vitro biopharmaceutical evaluation of solid dosage forms containing doxycycline. Brazilian Journal of Pharmaceutical Sciences 41: 451-458.
- Hadad GM, El-Gindy A, Mahmoud WM (2007) HPLC and chemometrics-assisted UV-spectroscopy methods for the simultaneous determination of ambroxol and doxycycline in capsule. Spectrochimica Acta 70: 655-663.
- Rufino JL, Fernandes FCB, Mayara SR, Pezza HR, Pezza L (2010) A simple spectrophotometric method for the determination of tetracycline and doxycycline in pharmaceutical formulations using chloramine-T. Eclética Química 35: 139-146.
- Leita EG (2005) Estabilidade: importante parâmetro para avaliar a qualidade, segurança e eficácia de fármacos e medicamentos. Dissertação, Mestrado em Ciências Farmacêuticas, Universidade Federal do Rio Grande do Sul, Porto Alegre.
- Silva KER, Alves LDS, Soares MFR, Passos RCS, Faria AR, et al. (2009) Modelos de Avaliação da Estabilidade de Fármacose Medicamentos para a Indústria Farmacêutica. Revista de Ciências Farmacêuticas 30: 129-135.
- International Conference on Harmonization (2003) ICH Guidance for Industry Q1A (R2), Stability testing guidelines: Stability testing of new drug substances and products. US Department of Health and Human Services Food and Drug Administration.
- Bajaj S, Singla D, Sakhuja N (2012) Stability Testing of Pharmaceutical Products. Journal of Applied Pharmaceutical Science 2: 129-138.
- Simon P, Veverka M, Okuliar J (2004) New screening method for the determination of stability of pharmaceuticals. International Journal of Pharmaceutics 270: 21-26.
- Malik A, Kumar V, Sunil R, Kumar T (2011) World Health Organization’s Guidelines for Stability Testing of Pharmaceutical Products. Journal of Chemical and Pharmaceutical Research 3: 892-898.
- Resolução - RDC Nº 45, de9 de agosto de (2012) Realização de estudos de estabilidade de insumos farmacêuticos ativos. Diário Oficial da União, Brasília, Brazil.
- Resolução RE nº 1, de 29 de julho de (2005) Guia para realização de Estudos de Estabilidade. Diário Oficial da União. Brasília, Brazil.
- Kogawa AC, Salgado HR (2013) Quantification of Doxycycline Hyclate in Tablets by HPLC-UV Method. J Chromatogr Sci 51: 919-925.
- Kogawa AC, Tomita LK, Salgado HRN (2012) Development and validation of a stability indicative turbidimetric assay to determine the potency of doxycycline hyclate in tablets. International Journal of Microbiology Research 4: 316-321.
- Kogawa AC, Salgado HRN (2013) Quantification of Doxycycline Hyclate in Tablets by Ultraviolet Spectrophotometric Method. World Research Journal of Pharmaceutical Research 1: 21-24.
- Nudelman NES (1975) Estabilidad de medicamentos. El Ateneo, Buenos Aires, Argentina.
- Lachman L, DeLuca P, Akers M (2001) Testes de estabilidade e fundamentos de cinética química. In: Lachman L, Lieberman HA, Kanig JL (eds.). Teoria e Prática na Indústria Farmacêutica. 2ndedn, Fundação Calouste Gulbenkian, Lisboa, Portugal.
- Campbell MJ, Machin D, Walters SJ (2007) Medical Statistics for Health Sciences. 4thedn, John Wiley & Sons Ltd, England.
- Vaucher LC, Paim CS, Lange AD, Schapoval EES (2010) Degradation Kinetics of Telithromycin Determined by HPLC Method. Journal of Chromatographic Science 48: 835-839.
- Kogawa AC, Salgado HRN (2017) Rifaximin Stability: A Look at UV, IR, HPLC, and Turbidimetry Methods. Journal of AOAC International.
 LOGIN
LOGIN REGISTER
REGISTER.png)