Research Article
A Meta-Analysis of the Efficacy of Immediate Release Methylphenidate to Reduce Hyperactivity in Children with Autistic Spectrum Disorder
Alison Margaret Bratt*, Bernadette Masanyero-Bennie and Stephen Patrick Kelley
Medway School of Pharmacy, The Universities of Kent and Greenwich at Medway, Central Avenue, Chatham Maritime, Kent, ME4 4TB, United Kingdom
*Corresponding author: Alison Margaret Bratt, Medway School of Pharmacy, The Universities of Kent and Greenwich at Medway, Central Avenue, Chatham Maritime, Kent, ME4 4TB, United Kingdom, Tel: +44 01634892944; Fax: +44 01634883927; E-mail: amb54@kent.ac.uk
Citation: Bratt AM, Masanyero-Bennie B, Kelley SP (2017) A Meta-Analysis of the Efficacy of Immediate Release Methylphenidate to Reduce Hyperactivity in Children with Autistic Spectrum Disorder. J Pharmacol Clin Trials 2017: 11-20. doi: https://doi.org/10.29199/JPCT.101014
Received Date: 05 July, 2017; Accepted Date: 11 August, 2017; Published Date: 28 August, 2017
Abstract
Psychostimulant medications, such as Methylphenidate (MPH) have become main stay treatments for Attention Deficit Hyperactivity Disorder (ADHD) symptoms, such as hyperactivity, impulsivity and inattention in children. Such symptoms frequently also co-exist in children with Autistic Spectrum Disorders (ASD), thereby complicating a differential diagnosis. The presence of ADHD-like symptoms in children with ASD can have a significant detrimental impact on learning and social interaction, and can limit the outcome of behavioural interventions to address areas of core ASD difficulties. Therefore, it is of clinical relevance and potential benefit to address the issue of effectiveness and tolerability/safety of stimulants such as methylphenidate in children with co-morbid diagnoses of ASD + ADHD.
This study performed a meta-analysis of three randomised controlled trials homogeneously measuring the effect of immediate release Methylphenidate (MPH) to reduce scores of hyperactivity in children with comorbid ADHD + ASD. The findings that IR-MPH produces an overall moderate significant benefit to reduce hyperactivity in ASD are discussed in relation to the risk/benefit question of medication use in this population.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) and Autistic Spectrum Disorders (ASD’s) are both neurodevelopmental conditions with symptoms emerging in early childhood, [1]. ADHD is characterised by increased levels of generalised activity, agitation, attentional difficulties and impulsivity [2-4]. ASD’s are characterised by deficits in engaging in reciprocal social interaction and with behavioural rigidity/lack of flexibility [2,3,5]. When considering the clinical diagnostic descriptors of ADHD and ASD, there is little in common in terms of their presentation, however, in clinical practice children with ASD displaying concomitant “ADHD-like” behaviours are commonly observed [2,6]. Indeed, recent data from a 2014 US survey of the diagnosis & treatment of ADHD showed that one in eight children with ADHD also had a concomitant diagnosis of ASD [7]. Section E of the diagnostic criteria for ADHD [8] does not allow a comorbid diagnosis of ASD and ADHD, though there are examples of ADHD being co-morbid with other frontostriatal disorders such as obsessive-compulsive disorder & conduct disorder [9]. The rationale has been that symptoms of ADHD in ASD are attributable to an ASD diagnosis. Which broaches the question “is a diagnostic comorbidity of ASD and ADHD justified?” Comorbidity could simply be the result of the broad diagnostic description of both disorders. ADHD symptoms in children with ASD appear superficially similar to ADHD symptoms in children without ASD and vice versa [8,10]. Based on the current diagnostic criteria an overlap exists making it difficult to distinguish them, but can or should these disorders be treated similarly? [11].
In order to address this, we must consider if the two disorders share aetiological and neuroanatomical commonalities? Many structural abnormalities have been found in the frontal lobe for both ASD [12,13] and ADHD but neuroimaging studies of autism and ADHD have shown general inconsistencies, making it difficult to determine relevant similarities and differences between the disorders [12,14].
Executive function deficits are common in both ASD and ADHD [15,16]. Executive functions are mediated by the prefrontal cortex [17] and involve domains of planning and goal directed acts, problem solving and strategy development, flexibility, persistence toward a goal state and self-awareness [18], with such functions being mediated through a network of neurons that interconnect on dendritic spines. The ASD- behaviours proposed to be related to executive dysfunction include, repetitive behaviours, lack of impulse control and cognitive flexibility/difficulty switching between tasks [19]. While in ADHD, response inhibition is regarded as the core deficit causing secondary deficiencies in other executive functions such as planning, working memory, and hyperactivity [18]. The literature highlights the complex nature of executive function dysregulation in neurodevelopmental disorders and the fact that individual subtypes of the disorders have an impact upon presentation of symptoms.
Data from epidemiological twin and family studies have provided evidence of strong genetic influences linked to the development of both ASD and ADHD. Both are neurodevelopmental disorders, with suggestions that ADHD results from a delay in brain maturation rather than a complete deviation from typical development [20]. The brain maturation hypothesis takes its evidence from the fact that ADHD symptoms sometimes tend to improve with age, with 32.4%-82.4% of children reported to show diminishing ADHD symptoms as they progress into adulthood [21]. Conversely, ASD is a life–long unremitting condition in which affected individuals show behavioural deficits throughout the developmental trajectory. Such differences between the disorders raises considerations which should be addressed regarding relative efficacy, duration of use and long-term safety of stimulant medication in children with ASD.
Standard drugs for treating ADHD are stimulant medications, such as Methylphenidate (MPH), and non-stimulants such as atomoxetine [22]. The National Institute of Clinical Excellence, (N.I.C.E.) Diagnostic & Clinical Guideline CG72 for ADHD states that stimulant medication should be considered as first line treatment (incorporated into a comprehensive care package) for children with severe symptoms, or those with moderate symptoms for whom non-drug interventions have failed (NICE, 2016). Considering the mechanism of action of methylphenidate, it is known to bind to the Neuronal Dopamine Transporter (DAT) with high affinity thereby blocking the inward transport of dopamine and prolonging the action of dopamine at its postsynaptic receptors in the prefrontal cortex [23]. MPH affects DA transport indirectly via the Vesicular Monoamine Transporter-2 (VMAT-2), located on intracellular vesicular membranes of monoaminergic neurones (which is known to remove cytoplasmic dopamine and noradrenaline into vesicles and control their storage and release) [24]. It is expected that, following a single administration of MPH a rapid and reversible increase in vesicular dopamine transport by the VMAT-2 takes place promoting removal of dopamine into vesicles for future release [25]. Augmentation of dopamine and noradrenaline in the prefrontal cortex is believed to then ameliorate executive dysfunction.
From existing literature, it is clear there are some similarities, but also major differences between ADHD and ASD in terms of the diagnostic criteria, presentation of symptoms, brain changes, affected neurotransmitters and treatment strategies. The question remains: Is it of clinical benefit to use stimulant medication in ASD even though the neurotransmitters (dopamine & noradrenaline), that are enhanced by stimulant medication are not known to be primarily dysregulated in ASD? Although clinical data showing positive short and long-term efficacy of MPH to reduce symptoms of hyperactivity in children with pure ADHD is convincing [20,26] there is still a lack of data regarding its long-term safety in children with ADHD [27]. Only a few studies have collected efficacy data on short term use of MPH on ADHD-like symptoms in children with a primary diagnosis of autism. It is the aim of this review and meta-analysis to clarify the efficacy of the immediate release preparation of methylphenidate to reduce hyperactivity in children with autistic spectrum disorder. This may help inform the clinical appreciation of the benefits/risk ratio that pharmacotherapy might have on such a vulnerable population.
Methods
Objectives
The study objectives were threefold: i) To perform a systematic review by searching several databases to identify relevant literature on the use of stimulant medication for treating ADHD-related symptoms in children with ASD. The search focused on studies that assessed the use of methylphenidate in children and adolescents, (3-14 years of age) as it is the first line medication for treating ADHD and ADHD symptoms in ASD within the UK. ii) To extract relevant data from randomized control studies, in order to perform a meta-analysis to determine the scope of effectiveness of methylphenidate to control problematic ADHD-like behaviours in children and adolescents with ASD iii) To conduct a concomitant appraisal of the tolerability of methylphenidate in this patient sub grouping.
A decision was made to concentrate this study only on the effectiveness of immediate release MPH as this comprised the greatest number of completely homogeneous studies which were amenable to comparison utilising the meta-analytical approach.
Inclusion criteria
This review involves an analysis of studies that focused on use of MPH in treating ADHD related symptoms in children and adolescents with ASD. Each study that met the pre-defined inclusion criteria was analysed and summarized within the following categories: a) Methods used - specifying rating scales employed, b) Aims of the study i.e., assessment of efficacy, tolerability, c) Duration and sample size, d) Results e) Limitations and conclusions. To assess the certainty of the evidence stated by each of the selected studies, the studies were critically appraised, taking into consideration the study design.
Search strategy
The following databases were searched: PsycINFO, PubMed-MEDLINE, Cochrane Controlled Trials and Web of Science. The search terms were as listed below:
Pervasive Developmental Disorders (PDD) / Methylphenidate / Hyperactivity / Children
Autism (Autistic Spectrum Disorders) / Methylphenidate / Hyperactivity / Children
Asperger’s Syndrome / Methylphenidate / Hyperactivity / Children
Pervasive Developmental Disorders PDD / Ritalin / Hyperactivity / Children
Autism (Autistic Spectrum Disorders) / Ritalin / Hyperactivity / Children
Asperger’s Syndrome / Ritalin / Hyperactivity / Children
Pervasive Developmental Disorders PDD / Concerta / Hyperactivity / Children
Autism (Autistic Spectrum Disorders) / Concerta / Hyperactivity / Children
Asperger’s Syndrome / Concerta / Hyperactivity / Children
Pervasive Developmental Disorders (PDD) / Stimulant / Hyperactivity / Children
Autism (Autistic Spectrum Disorders) / Stimulant / Hyperactivity / Children
Asperger’s Syndrome / Stimulant / Hyperactivity / Children
The search was completed in January 2012. The reference lists for the studies meeting the inclusion criteria were reviewed to identify any additional studies that also met the inclusion criteria.
A follow up search was conducted in May 2017 utilising the same search criteria, which uncovered four additional studies. These studies were of topic relevance, but could not be included in the meta-analysis for the reasons given in the inclusion & exclusion criteria section below.
Inclusion and exclusion criteria
To be included, studies had to have been written in English. The subjects had to be aged between 3-18 years. Though stimulant medications are licensed for children over the age of 6 within the UK, we felt that it was vital for pre-school aged children to be included as there have been reports of use in children under 6 years [28]. The subjects had to meet the diagnostic criteria for ASD (difficulties in social communication & inflexibility of behaviour) in accordance with the Diagnostic & Statistical Manual of Mental Disorders DSM5, (2013) or the International Classification of Diseases-11, (2012). The presence of any other co-morbidity led to the study being excluded to limit dependent variables and avoid the results of the studies being explained by other contributing factors. Other comorbidities commonly existent with ASD include affective disorders, depression and anxiety and Obsessive Compulsive Disorder (OCD). Participants included in our analyses had only diagnoses of ASD + ADHD to allow as homogenous a set of participants as was feasible.
For ease of comparison, only studies utilising Immediate Release Methylphenidate (IR-MPH) were included. This was decided since IR- MPH is the most widely used stimulant drug in children within the UK and also so as to avoid any variability in dose conversions of other stimulant drugs. Insufficient reports of extended release formulations of MPH existed of sufficient homogeneity to be able to perform meta-analysis. Case reports were excluded as their methodology was not considered rigorous enough.
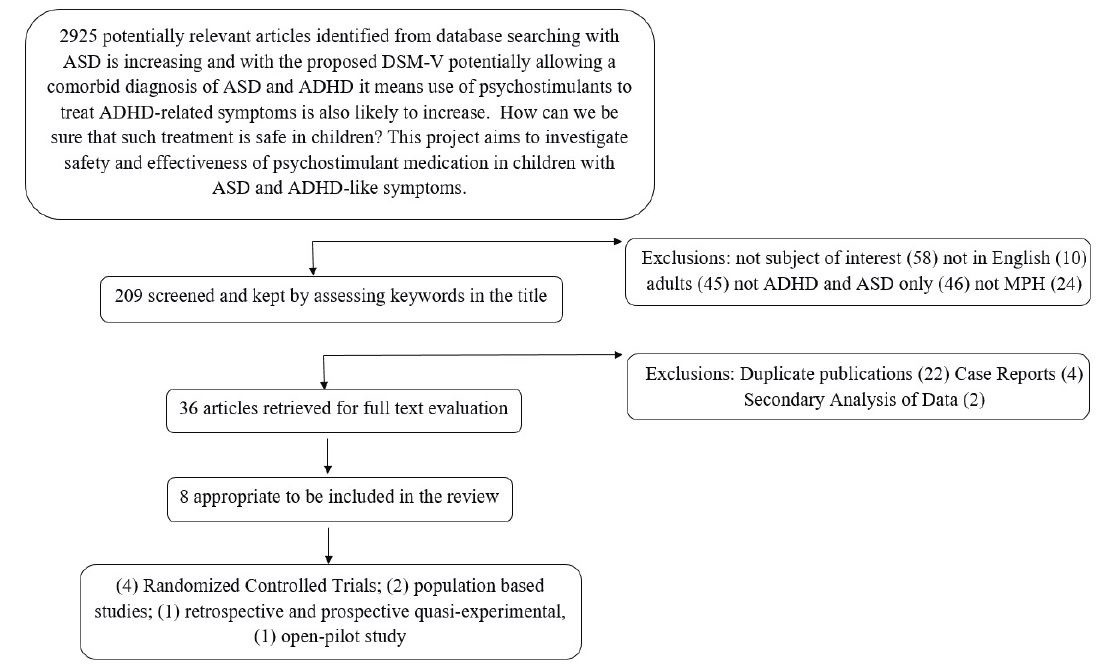
An additional four studies were found in the follow up literature search conducted in 2017. These were considered of interest for synthesis of findings and have been included in the discussion, but could not be included in the meta-analysis for the following reasons: Maia et al., [29] presented a secondary analysis of the RUPP study data which had already been included in our analysis; Marco et al., [30] presented data using immediate release MPH however the study design was not homogeneous with our analyses; and Madras et al., McCracken et al., [31,32] both conducted trials with extended release methylphenidate in subjects with ASD + ADHD (Figure 1).
|
Figure 1: Flowchart depicting the strategic process utilised in the selection and exclusion of relevant research studies to be included in the systematic review and meta-analysis. |
Study selection & data extraction
Eight potentially relevant studies were identified and the full text articles were obtained and read in full. The studies were then grouped within their respective study design and summarized in tabular format for comparison. Information on study design, sample size, sex ratio, and research tool used to assess ADHD symptoms, dose, efficacy and tolerability of MPH was extracted independently by two of the research team. Four of the studies were randomized control trials; [4,32-34] One study was a retrospective and prospective quasi-experimental study design [5]; Two population based studies were also reviewed [11,35] in addition to one open pilot study [36].
Data analysis
The meta-analytical style of data analysis tends to be used in the context of systematic reviews of Randomized Control Trials (RCT’s) and this is why it was chosen for this comparative study. The design of these RCTs is usually based on patients being randomly allocated to one of two different but parallel treatment groups [37]. All four of our RCTs which were selected were cross-over trials, whereby each patient acted as his/her own control, and received two or more treatments; and the sequence of treatment was randomized. These criteria assured complete homogeneity of data and meant that the four studies selected were amenable to analysis utilising the meta-analytical approach. Data on treatment benefits i.e. those classed as responders and those classed as non-responders within the RCTs was extracted, and comparatively analysed [38].
Results
In order to maintain and ensure homogeneity of data, a decision was made to focus only on the four Randomized Control Studies (RCTs) which are summarized in table 1 below. The study design and doses used in the four studies were similar, all being (RCT)s with cross-over trials, whereby each patient acted as his own control and received two or more treatments; the sequence of treatment being randomized. A meta-analysis (fixed model), was performed based on the teacher/practitioner ratings on the Aberrant Behaviour Checklist (ABC hyperactivity subscale) following treatment with placebo or methylphenidate. The study by McCracken et al., [32] was excluded as it did not quantitatively report on how many children had responded or not responded to MPH treatment.
Study Characteristics:
Table 1: Summary of the selected randomized control trials of IR-MPH in treatment of ADHD-like symptoms in children/adolescents with ASD. ABC: Aberrant Behaviour Checklist; AD: Autistic Disorder, AS: Asperger’s Syndrome, ASD: Autism Spectrum Disorders; b.i.d: Twice daily dosing; CPRS: Child Psychiatric Rating Scale; CTRS: Conners Teacher Rating Scale [38] RUPP: Research Unit on Paediatric Psychopharmacology, t.i.d: Three times daily.) a Initially 72 subjects enrolled in the test dose phase, 6 exited owing to adverse effects b At high dose n=50. L=low dose, M= Medium dose, H=High dose. *Approximate dose ranges based on age and body weight. |
The total number of participants showing positive response to IR-Methylphenidate across all studies ranged from 47% [32,33] to 59%. This indicates that positive response rates for children with ASD appear to be lower than those quoted for children with a sole diagnosis of ADHD. Also, the number of children discontinuing from the study ranged from 6% [33,34] to 22% (Figure 2).
|
Figure2: Histogram showing the range of response to immediate release methylphenidate across the studies. The % number of participants who were classed as responders, (dark grey bars) and the % number of participants who discontinued treatment, (light grey bars) during the studies. |
Adverse event profile of IR-MPH
The adverse effects experienced were also considered and the four most frequent adverse effects following treatment with MPH reported in the RUPP study were; decreased appetite, difficulty falling asleep (insomnia), irritability and gastrointestinal discomfort. The adverse effect profiles of the other 3 studies were evaluated using the RUPP study as the control measure. The number of subjects experiencing each of the mentioned side effects was examined from each of the four studies. The Quintana study represented adverse events as percentage occurrence, with no mention of the number of subjects. The RUPP study and the Ghuman study broke down the side effects into those experienced at low dose (RUPP: 2.5-5mg; RUPP: 5-10mg) and those experienced at high dose (RUPP: 10-20mg; Handen: 10-20mg). The Ghuman study only gave the adverse effect profile without breaking down into low dose and high dose (2.5-20mg), so it impossible to know how many individuals had experienced the adverse-effect.
Efficacy if IR-MPH on hyperactivity
Three studies Quintana et al. [38], RUPP [32], Handen et al. [21] looked at mean baseline and post treatment with MPH rating on the ABC-Hyperactivity Scale. These results were also represented graphically to show whether treatment with MPH improved hyperactivity in the subjects (Table 2 and Figure 3). These results are based on the high dose for the RUPP study (10-20mg daily) and for the Handen study (1.2-1.8mg/kg daily~10-20mg) to closely resemble the dose administered in the Quintana study (20-40mg daily), in order to achieve homogenisation of data.
Table 2: Summary of the mean ratings on the ABC-hyperactivity subscale following treatment with placebo or Immediate-Release (IR) Methylphenidate across all three studies. |
|
Figure3: Teacher/Practitioner Rated ABC-Hyperactivity subscale [37] at baseline and following treatment with immediate release IR-MPH. Histogram bars show mean ABC hyperactivity score +/- standard error of the mean. Participant numbers per study are depicted below each studies name. |
Meta analysis of efficacy of IR-MPH on hyperactivity measure
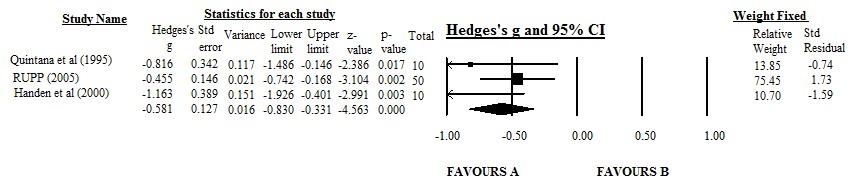
We utilised the Teacher rated mean ABC-Hyperactivity subscale score, which was one of the primary outcome measures of three of the RCTs Quintana et al. [38], RUPP [32], Handen et al. [21] as our outcome indicator. A meta-analysis was carried out using a trial of the Comprehensive Meta-Analysis software (www.meta-analysis.com) to compare the placebo versus IR-MPH effect and the results are represented as a forest plot in figure 4. Effect sizes for the measures in each study were expressed as Hedges’s g value. Hedges’s g uses n-1 to calculate standard deviation and is considered accurate since it allows for an adjustment for small sample size. These effect sizes were computed by taking the mean on the ABC following MPH minus the mean of the placebo divided by the pooled standard deviations. Studies were weighed according to the number of participants included. The fixed model was used as the studies were almost functionally similar and the goal of the meta-analysis was to compute the common effect size for the identified studies.
|
Figure 4: Forest Plot depicting the range of effect sizes relative to the mean for the three studies compared. Hedge’s g and 95% Confidence Interval (CI) are depicted according to study name. Individual box point symbols indicate the effect size for each study, while the horizontal line through each box gives the 95% CI. The diamond gives the average CI for all three studies. A= Methylphenidate, B= Placebo. |
The lines on the Forest plot represent each study plotted according to the Standardised Mean Difference (SMD), which is the average score of participants in the intervention group and the average of participants in the control group. The black diamond at the bottom of the graph shows the average effect size of all three studies. From the Forest plot, all lines fall on the left-hand side of the graph indicating that the participants receiving the intervention (immediate release methylphenidate) reported larger changes than participants receiving the control condition. The black diamond depicts the overall conglomerated effect size, and it sits between 0 and -1 showing that the effect size of the three trials is approximately -0.65 which can be classed as a moderate effect. Therefore, immediate release methylphenidate pharmacotherapy displays moderate efficacy to reduce hyperactivity scores in children with concomitant diagnoses of ADHD & ASD.
Discussion
The presence of hyperactivity, impulsivity and inattention in ASD has been commonly observed leading to the use of stimulant medication in children with ASD to target such symptoms. This study set out to determine the effectiveness and safety of stimulant medication when employed in this select population. Initially a systematic review was performed which yielded eight studies of which four were RCTs. To our knowledge, to date there has not been a study that has combined and quantified current evidence from independent papers on effectiveness of stimulant medication when used in children with ASD presenting with ADHD symptoms. We set out to perform a meta-analysis, using the RCTs from the literature review as a way of combining and quantifying current evidence on effectiveness of immediate release (IR)-MPH.
One of the primary outcomes for three of the RCTs Quintana et al. [38], RUPP [32], Handen et al. [21] was the teacher/practitioner rated hyperactivity on the ABC scale. The placebo and following treatment with MPH results were extracted and represented graphically with standard error bars. The results showed that there was some difference between treatment with placebo and MPH. To assess the effect of MPH within the three studies further, a meta-analysis was performed using the same results. The meta-analysis compared placebo and MPH effect in reduction of hyperactivity in children, and MPH was found to be superior to placebo with summary effects of -0.581 (95% CI -0.830 to -0.331) based on Hedges’s g index. The negative values indicate superiority of treatment with MPH over placebo in decreasing hyperactivity. This provided preliminary evidence to support short term effectiveness of MPH in children with ASD who also present with ADHD symptoms. Our results are in line with a recent systematic review by Siegel and Beaulieu [39] that quoted the evidence available on use of MPH in treatment of hyperactivity as promising. It is important to note that our findings are best considered preliminary as the results show reduction in hyperactivity only and are based on a small sample size (data from three RCTs only). The ratings on the ABC-hyperactivity were carried out by teachers in two of the studies [31,32] and in the third study [34] they were carried out by psychiatrists (practitioners). In all the studies, the teachers/practitioners were blind to the doses administered but it is hard to rule out the subjective element of rating scales which might have influenced the results. For the Quintana study, the ratings were carried out on one day a week only following a three-hour intensive simulated structured classroom session, this potentially did not allow enough time for environmental adaptations for the participants. There is no mention of the frequency or the nature of the environment the participants were assessed in the [32,33]. There was no inclusion of unpublished articles and the total sample size was small. There is a need for a larger meta-analysis to confirm these results as more study data become available.
It is prudent to note that the vast majority (85%) of the participants of the studies included in this analysis were boys, with only 15% girls (Total pooled N=103). This type of gender disparity in the prevalence of autism has been noted numerous times in the literature. A most recent American survey of prevalence & characteristics of ASD [40] found ASD prevalence was higher for boys (23.6 per 1,000) than girls (5.3 per 1,000); equating to a 4.45 (male): 1 (female) ratio, very similar to our findings. It is useful to consider whether IR-MPH would have similar benefit in girls with comorbid ASD/ADHD? Considering the paucity of actual clinical data from females, we cannot be exact in our estimations. However, very recent study surveying 566 males and 113 females with ASD concluded that there were no gender differences in ASD symptom severity [41]. If we consider that characteristics and severity of the presentation of ASD may impact upon pharmacological treatment efficacy and behavioural control, this may allow us to surmise that MPH treatment could be equi-effective at reducing some symptoms of ADHD, such as hyperactivity, in both genders?
To further assess effectiveness and safety, data on response rate, discontinuation rate and adverse-effects was also extracted from the RCTs. One of the RCTs [32] did not have quantitative data on response rate, so it was excluded. The combined percentage response to MPH for the remaining three RCTs [33,34] and was 50.5% and the discontinuation rate was 17.7% due to adverse drug effects, which gave some indication on safety and tolerability of MPH in ASD. There was a general increase in subjects experiencing adverse effects as the dose was increased, but it was unclear why a high proportion of subjects from the [33] experienced an adverse effect following placebo treatment. None of the RCTs had compared response and safety of MPH in an ADHD only group in comparison to ASD combined with ADHD symptoms. A retrospective and prospective study by (NICE, 2008) [4] was the only study that did a comparison of stimulant medication in children with ASD combined with ADHD symptoms and ADHD alone. The study showed improvements in target symptoms in both groups without any significant differences in the magnitude of improvement between the groups. Sleep difficulties were among the commonly reported side effects and there were no statistically significant differences in side-effect profiles of the two groups. The study was done over a short period of time (1-6 months duration), hence no indication of long-term effectiveness of MPH.
The RCTs did not evaluate long term effectiveness as they were carried out over a short period of time, but two retrospective studies [11,36] evaluated effectiveness and tolerability of stimulant medication in children and adolescents. The study by [11] concluded that stimulants appeared to be ineffective and poorly tolerated as adverse effects were observed in 57.5% of the trials evaluated. On the other hand, the study by [36] concluded that there was some improvement in the target symptoms of hyperactivity, impulsivity, disinhibition and inattention, and side effects were not sustained throughout treatment episodes. It remains unclear whether stimulant medications are beneficial in the long term, since most clinical trials are of short duration and do not include a maintenance data collection phase. It is quite alarming that even for ADHD only; an indication for which there has a far more established use of stimulant medication; there is limited and inconsistent evidence for positive clinically relevant long-term effects of stimulant medication. Long term follow-up data of the MTA study [35] (the biggest study to date on ADHD) failed to provide support for long term advantages of medication treatment and symptom control [42]. Discontinuation rates for immediate-release methylphenidate in ADHD have been reported to be in the region of 36-51% within one year of treatment initiation and are due to adverse drug effects, non-compliance or adherence issues [28].
From the collective information that was evaluated in this study, the effectiveness of IR-MPH in the short term is promising. These findings should be viewed with caution as they are based on hyperactivity only. However more recent studies have indicated that other behaviours may be improved by methylphenidate treatment. [20] completed a secondary analysis of the RUPP data (already included in this meta-analysis assessing hyperactivity), and showed evidence that MPH has positive effects on social behaviour (joint attention, self-regulation and affective state) utilising a 4-week crossover placebo vs increasing doses of MPH over 1 week in 33 participants with ASD + ADHD. Also, when considering use of extended release formulations of MPH [27] demonstrated positive dose-related effects to decrease hyperactivity, irritability, lethargy and stereotypy in a 6 week trial (N=27 participants). Social behaviours were improved and aspects of impulsivity, inattention and oppositional behaviours were attenuated by extended release MPH in a further study conducted by McCracken et al. [31]. These studies could indicate that stimulant treatment in at least a sub-group of young children/young people with ASD + ADHD could have more of a holistic beneficial effect?
The adverse drug effects which are apparent from this study are likely to be an unpredictable drawback in the use of MPH. The most commonly reported adverse effects of MPH in ASD are appetite suppression, sleep disturbances and irritability [12,31-35,43,44] but why may adverse effects appear to be more frequent and severe in ASD?
The frequency and severity of adverse effects could have neurochemical, genetic or behavioural underpinnings. From a behavioural perspective side effects are likely to be partially due to the augmentation of problems already present in ASD as a result of treatment with MPH. Sleep disturbances are highly frequent in ASD with prevalence rates ranging from 50-80% [45] they are multifactorial with a variety of mechanisms purported. Hypersensitivities to sound, light, certain textures or fabrics and abnormalities in melatonin release (amount or timing) may all contribute to sleep disturbances [31]. MPH is known to increase extracellular levels of DA by disrupting the function of DAT, thus enhancing DA signalling in reward and motor pathways [46]. This results in promotion of arousal and physical activity, further impeding sleep in children with ASD. Inadequate sleep is associated with exacerbation of core ASD symptoms such as deficits in social skills, increased aggression, tantrums as well as emotional difficulties which are equally as undesirable as the ADHD-like symptoms.
Children with autism are reported as having selective eating habits and refuse many foods as they have a strong preference for sameness in colour and texture [46]. This has been linked to tactile and olfactory hypersensitivity which results in children with ASD having difficulties with food textures or smells [39]. Stimulant medications such as MPH are associated with a decrease in appetite. The combination of appetite decreases and selective eating habits is likely to result in inadequate nutrition and reduction in caloric intake which in the long term will lead to more pronounced growth deficits in this vulnerable population.
A pharmacogenetics study conducted by Marco et al. [30] interestingly addresses the large variation in both response and tolerability to stimulant medication in the ASD + ADHD population. This was quite a large study (N=64) measuring hyperactivity in a blind cross over design 4-week study with 3 doses of MPH. The study showed 25% of the participants with much improved hyperactivity, which is in agreement with our meta-analysis. The McCracken study [24,33] also reports a 22% discontinuation rate due to side effects, and this showed high correlation with variants in several genes related to elements of monoaminergic modulation (such as the dopamine transporter protein, and dopamine receptors, D1/D3/D4). This study shed fascinating light on the possibility that there may be a greater sensitivity to the pro-dopaminergic effects of stimulants such as methylphenidate within the ASD population.
Do the short-term benefits of MPH outweigh long term risks? This question is beyond the scope of this paper but highlights the need for further investigations of the effects of MPH in the long term. It also highlights the need for pre-medication assessments that can aid clinicians in the identification of those children who are likely to experience severe adverse effects from treatment. There is also a need for a clearer understanding of the aetiology, neurobiology and neurochemistry of ASD in order to enable selective pharmacological treatments to be employed that target core behavioural deficits.
Conclusion
The aim of this research was to investigate the effectiveness of IR-Methylphenidate stimulant medication in children with ASD who present with ADHD symptoms and also to consider its tolerability profile. Results of the study indicated that short-term use of IR-MPH has moderately beneficial effects in reducing hyperactivity in children with ASD. These results are best considered preliminary as they are based on three RCTs only, all with quite small sample sizes. There is a need for further analysis as more study data on medication used to manage the aforementioned target symptoms becomes available. Safety of MPH appears to be a real issue as a high number of adverse effects were experienced within the studies, which were severe enough to lead to frequent treatment discontinuation. It is interesting to consider if there is/are any particular characteristic(s) of the ASD phenotype which may predict higher propensity to experience an intolerable side effect profile with stimulant medication, however, further investigations are required to confirm this.
Future Research
Future investigations may shed light on what factors predict side effect profile in this population. It might be of value to evaluate the effects of subtypes within ASD as results from the study by Cermak et al. [11] indicated that children with Asperger’s disorder were more likely to positively respond to treatment than individuals with autistic disorder [37] also stated that the subtype of ASD appeared to have an influence on response or tolerability to MPH as children with Asperger’s disorder or PDD-NOS tolerated MPH better. Obviously, a study of individualized treatment and dose titration for each child must be considered alongside other conceivable variables affecting overall outcome. There is also a need to identify the clinical and biological variables that can predict which children would respond to treatment with stimulant medication and hence avoid exposure in those children who would otherwise develop adverse effects.
There is also a need for longitudinal studies to assess the efficacy of methylphenidate available in more extended release formulations as an extension of this work. With a need to study the effects of MPH over a longer period by conducting RCT’s with inherent maintenance phases. This might help to identify if there is any correlation between typical ASD symptoms and severity of adverse medication effects commonly observed.
References
- Aagaard L, Hansen, EH (2011) The occurrence of adverse drug reactions reported for Attention Deficit Hyperactivity Disorder (ADHD) medications in the pediatric population: a qualitative review of empirical studies. Neuropsychiatr Dis Treat 7: 729-744.
- DSM-5 (2013) Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5). American Psychiatric Association.
- ICD11 (2012) World Health Organization: The International Classification of Diseases 11th Revision.
- National Institute for Health and Clinical Excellence (NICE) (2008) Attention deficit hyperactivity disorder: diagnosis and management. Clinical guideline [CG072] Updated 2016.
- National Institute for Health and Clinical Excellence (NICE) (2011) Autism spectrum disorder in under 19s: recognition, referral and diagnosis. Clinical guideline [CG128].
- Mayes SD, Calhoun SL, Aggarwal R, Baker C, Mathapati S, et al. (2012) Explosive, oppositional and aggressive behavior in children with autism compared to other clinical disorders and typical children. Res Autism Spect Disord 6: 1-10.
- Zablotsky B, Bramlett MD, Blumberg SJ (2017) The co-occurrence of autism spectrum disorder in children with ADHD. J Atten Disord.
- Barkley RA (2000) Genetics of childhood disorders: XVII. ADHD, Part 1: The executive functions and ADHD. J Am Acad of Child Adolesc Psychiatry 39: 1064-1068.
- Aman MG, farmer CA, Hollway j, Arnold LE (2008) Treatment of inattention, overactivity and impulsiveness in autism spectrum disorders. Child Adolesc Psychiatr Clin N Am 17: 713-738.
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, et al. (2010) Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 49: 229–238.
- Cermak SA, Curtin C, Bandini LG (2010) Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc 110: 238-246.
- Cortese S, Castelnau P, Morcillo C, Roux S, Bonnet-Brilhault F (2012) Psychostimulants for ADHD-like symptoms in individuals with autism spectrum disorders. Expert Rev Neurother 12: 461-473.
- Conners CK, Sitarenios G, Parker JDA, Epstein JN (1998) Revision and restandardization of the conners teacher rating scale: factor structure, reliability and criterion validity. J Abnorm Child Psychol 26: 279-291.
- Mayes SD, Calhoun SL, Mayes RD, Molitoris S (2012) Autism and ADHD: Overlapping and discriminating symptoms. Res Autism Spect Disord 6: 277-285.
- Di Martino A, Melis G, Cianchetti C, Zuddas A (2004) Methylphenidate for pervasive developmental disorders: safety and efficacy of acute single dose test and ongoing therapy: an open-pilot study. J Child Adolesc Psychopharmacol 14: 207-218.
- España RA, Scammell TE (2011) Sleep neurobiology from a clinical perspective. Sleep 34: 845-858.
- Frazier JA, Biederman J, Bellordre CA, Garfield SB, Geller DA, et al. (2001) Should the diagnosis of attention-deficit/ hyperactivity disorder be considered in children with pervasive developmental disorder? J Att Disord 4: 203-211.
- Gargaro BA, Rinehart NJ, Bradshaw JL, Tonge BJ, Sheppard DM (2011) Autism and ADHD: how far have we come in the comorbidity debate? Neurosci Biobehav Rev 35: 1081-1088.
- Ghuman JK, Aman MG, Lecavalier L, Riddle MA, Gelenberg A, et al. (2009) Randomized, placebo-controlled, crossover study of methylphenidate for attention-deficit/hyperactivity disorder symptoms in preschoolers with developmental disorders. J Child and Adolesc Psychopharmacol 19: 329-339.
- Groenman AP, Schweren LJ, Dietrich A, Hoekstra PJ (2017) An update on the safety of psychostimulants for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Drug Saf 16: 455-464.
- Handen BL, Johnson CR, Lubetsky M (2000) Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord 30: 245-255.
- Happe F, Booth R, Charlton R, Hughes C (2006) Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn 61: 25-39.
- Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, et al. (2009) Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord 39: 395-404.
- Kim SJ, Shonka S, French WP, Strickland J, Miller L, et al. (2017) Dose-response effects of long-acting liquid methylphenidate in children with Attention Deficit Hyperactivity Disorder (ADHD) and autism spectrum disorder (ASD): a pilot study. J Autism Dev Disord 47: 2307-2313.
- Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, et al. (2009) Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry 65: 46-54.
- Levy SE, Mandell DS, Schultz RT (2009) Autism. Lancet 374: 1627-1638.
- van den Ban E, Souverein P, Swaab H, van Engeland H, Heerdink R, et al. (2010) Trends in incidence and characteristics of children, adolescents and adults initiating immediate- or extended-release methylphenidate or atomoxetine in the Netherlands during 2001-2006. J Child Adolesc Psychopharmacol 20: 55-61.
- Lockner DW, Crowe TK, Skipper BJ (2008) Dietary intake and parents' perception of mealtime behaviors in preschool-age children with autism spectrum disorder and in typically developing children. J Am Diet Assoc 108: 1360-1363.
- Maia CR, Cortese S, Caye A, Deakin TK, Polanczyk GV, et al. (2017) Long term efficacy of methylphenidate immediate-release for the treatment of childhood ADHD. J Atten Disord 21: 3-13.
- Marco EJ, Hinkley LB, Hill SS, Nagarajan SS (2011) Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res 69: 48-54.
- Madras BK, Miller GM, Fischman AJ (2005) The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 1397 -1409.
- McCracken JT, Badashova KK, Posey DJ, Aman MG, Scahill L, et al. (2014) Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J 14: 295-302.
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, et al. (2009) The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 48: 484-500.
- MTA Cooperative Group (1999) A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Multimodal treatment study of children with ADHD. Arch Gen Psychiatry 56: 1073-1086.
- Nickels KC, Katusic SK, Colligan RC, Weaver AL, Voigt RG, et al. (2008) Stimulant medication treatment of target behaviors in children with autism: a population-based study. J Dev Behav Paediatr 29: 75-81.
- Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, et al. (2013) Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J Child Adolesc Psychopharmacol 23: 337-351.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Front Matter. In: Introduction to Meta-Analysis. John Wiley & Sons Ltd. Chichester UK.
- Quintana H, Birmaher B, Stedge D, Lennon S, Freed J, et al. (1995) Use of methylphenidate in the treatment of children with autistic disorder. J Autism and Dev Disord 25: 283-294.
- Siegel M, Beaulieu AA (2012) Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. J Autism Dev Disord 42: 1592-1605.
- Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, et al. (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years-autism & developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ 65: 1-23.
- Mussey JL, Ginn NC, Klinger LG (2017) Are males and females with autism spectrum disorder more similar than we thought? Autism 21: 733-737.
- Rommelse N, Franke B, Geurts HM, Hartman CA, Buitelaar JK (2010) Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 19: 281-295.
- RUPP Autism Network (2005) Randomized, controlled crossover trial of mph in pervasive developmental disorders with hyperactivity. Arch of Gen Psychiatry 62: 1266-1274.
- Rutter M, Bishop D, Pine D, Scott S, Stevenson J, et al. (2008) Rutter’s Child and Adolescent Psychiatry. (5th Edition), Oxford 5thedn. Blackwell Publishing.
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE (2002) Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci 22: 8705– 8710.
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, et al. (2007) Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Nati Acad Sci USA, 104: 19649-19654.
 LOGIN
LOGIN REGISTER
REGISTER.png)