Research Article
Retrospective Review of Survival Outcomes in Glass and Resin Y-90 Microsphere Treatment of Hepatic Malignancies
Sarah DeBacker1, Jill Jones2, Joseph Vavricek3, Saad Iqbal4, Suzanne L Hunt5, Jo Wick5, Carissa Walter2, Jacqueline Hill2, and Zachary Collins2*
1Department of Radiology, Beth Israel Deaconess Medical Center, Boston, MA, USA
2Department of Radiology, University of Kansas Medical Center, Kansas City, KS, USA
3Mercy Medical Center, Des Moines, IA, USA
4United Northeast Radiology, Kingwood, TX, USA
5Department of Biostatistics, University of Kansas Medical Center, Kansas City, KS, USA
*Corresponding author: Collins Z, Department of Radiology, University of Kansas Medical Center, Kansas City, KS 66160, USA, Tel: +1 9136695234; E-mail: zcollins@kumc.edu
Citation: DeBacker S, Jones J, Vavricek J, Iqbal S, Hunt SL, et al. (2018) Retrospective Review of Survival Outcomes in Glass and Resin Y-90 Microsphere Treatment of Hepatic Malignancies. J Interv Radiol Nucl Med 2018: 1-9. doi:https://doi.org/10.29199/IRNM.101018
Received: 28 December, 2017; Accepted: 24 January, 2018; Published: 10 February, 2018
Abstract
Purpose: To compare survival outcomes of patients with unresectable hepatic malignancies treated with glass or resin Yttrium-90 (Y-90) Transarterial Radioembolization (TARE).
Materials and methods: This retrospective study included patients with unresectable Hepatocellular Carcinoma (HCC), metastatic Colorectal Carcinoma (mCRC), and other metastatic liver disease treated with Y-90 between January 1, 2008 and August 31, 2015. Mean time-to-death and survival outcomes were compared between glass and resin groups and by Portal Vein Invasion status (PVI) for patients with HCC. Kaplan-Meier curves, log-rank tests, and Hazard Ratios (HRs) were used to describe survival between treatment and primary pathology groups.
Results: One-hundred fourteen patients were included - 59 patients received glass treatment, while 55 received resin. Forty-eight patients (42.2%) had primary HCC, 38 (28.9%) had mCRC, and 33 (28.9%) had other hepatic metastases. Patients treated with resin had a 44% estimated reduction in risk of death compared to glass (HR=0.56, 95% CI=0.32-0.96; p=0.03). Differences in HCC patients’ one-year survival were statistically insignificant, even when examined by PVI status, although mean survival times were greater for resin treatments (270.4 days (SE=24.3) for resin treatments v. 231.0 days (SE=21.2) than for glass (HR=0.54, 95% CI=0.25-1.19). Similarly, mean survival times for patients with mCRC, and other liver pathologies were not statistically significantly different between glass and resin treatment groups (mCRC: HR=0.40, 95% CI=0.13-1.19; other pathologies: HR=1.07, 95% CI=0.36-3.18).
Conclusion: Patients who received treatment with resin survived longer than those who received glass. However, when examined by liver disease pathology, there were no statistically significant differences in one-year survival between treatment groups.
Keywords: Hepatocellular Carcinoma (HCC); Radioembolization; Y-90
Introduction
Primary and secondary hepatic malignancies are common and often present at an advanced stage when limited curative treatment options are available [1]. One promising treatment therapy, particularly for unresectable hepatic neoplasms, is endovascular radioembolization, including Transarterial Chemoembolization (TACE), Transarterial Embolization (TAE), and ablative therapy. However, compared to the other therapies, endovascular radioembolization is unique in its ability to deliver high radiation doses directly to tumors via micron-sized embolic particles, loaded with yttrium-90 (Y-90) radioisotopes, which penetrate 2.5 mm to 1.1 cm of the surrounding tissue [2-4]. Compared to external beam radiation in which normal liver parenchyma is intolerant to tumorocidal radiation doses, radioembolization has been shown to decrease collateral damage by providing targeted focal radiotherapy [5].
Radioembolization with Y-90 can be delivered via glass or resin microspheres, which are different in size, radioactivity, and dosimetry methods. First, glass microspheres (20-30 µm) are often smaller than resin (20-60 µm), but have higher radioactivity of 2500 Bq per sphere compared to 50 Bq per sphere for resin the time of calibration [6,7]. In addition, glass microspheres are structurally embedded with Y-90 radioactivity, whereas resin microspheres are surface-coated, resulting in a lower density of radioactivity. Therefore, more resin microspheres are deposited per given dose, resulting in a higher embolic effect for resin compared to glass microspheres with each administration [2,7]. For example, a three GBq vial of microspheres contains 1.2 million glass spheres compared to 40-80 million resin spheres [2]. Finally, glass microspheres are currently FDA approved for the treatment of unresectable HCC, while resin microspheres are FDA approved for the treatment of hepatic mCRC [6,7]. As a result of these differences, the two Y-90 treatments may expose the liver to varying amounts of radiation and embolization-induced ischemia, which could alter their efficacy and tolerability [8].
While both glass and resin microspheres have independently demonstrated efficacy in increasing survival for both primary HCC and metastatic liver disease, there is limited and conflicting research directly comparing survival outcomes between the two types of microspheres [3,4,7,9-12]. A recent retrospective study of 77 patients with HCC documented no significant survival differences for patients who received glass or resin treatments (p=0.77) [13]. However, when further examining HCC patients with PVI, a retrospective analysis of 90 patients reported a survival benefit of glass over resin microspheres (p<0.001) [14]. Conversely, a study of 28 patients with unresectable mCRC suggested a survival benefit for patients treated with resin compared to glass microspheres (p=0.0175), but this was only evident in the second year after treatment [8]. Given these conflicting findings, additional studies comparing the two treatments are warranted to further investigate survival outcomes, particularly for different liver pathologies. Therefore, the purpose of this study was to compare survival outcomes among patients with primary and secondary hepatic malignancies who received resin and glass Y-90 treatments. This study also sought to determine if there were any significant differences in reported complications between the treatment groups.
Materials and Methods
Participants
A retrospective study was conducted on consecutive patients with clinical evidence of unresectable HCC, mCRC, and other metastatic disease to the liver (such as carcinoid or breast metastases) who were treated with glass (known as TheraSphere® manufactured by BTG Biocompatibles Ltd, Farnham, United Kingdom) or resin (known as SIR-Spheres® manufactured by Sirtex Medical, Sydney, Australia) Y-90 microsphere radioembolization at a single institution between January 1, 2008 and August 31, 2015. All study activities were approved by an institutional review board and waiver of consent was obtained. One hundred thirty-one patients met initial inclusion criteria. Patients were excluded from analysis if they had missing pre-treatment or follow-up imaging data (n=14) and for receiving combination TACE treatment (n=3). Institutional Review Board (IRB) approval was obtained to review electronic medical records and the Picture Archiving and Communication System (PACS) for patient demographic, clinical, treatment, imaging, and survival information. Study data were collected and managed using REDCap electronic data capture tools [15].
At our institution, glass microspheres were utilized as the primary form of Y-90 treatment from 2008-2012. In 2012, Y-90 use gradually shifted to incorporate resin microspheres at the discretion of the treating interventional radiologist. As part of the clinical administration, all patients underwent hepatic angiograms approximately four weeks prior to treatment to map the hepatic arterial system, identify anatomic variants, determine tumoral blood supply, and embolize any vessels that could allow microspheres to enter the gastrointestinal tract. To assess safety of the upcoming Y-90 procedure, Macro Aggregated Albumin (MAA) was delivered via the hepatic artery, followed by the use of Single-Photon Emission Computed Tomography (SPECT) gamma imaging to detect shunting of particles through large intra-tumoral arteriovenous shunts into the gastrointestinal or pulmonary systems [7]. Once the procedure was deemed safe, patients returned within approximately 4-6 weeks for catheter-directed angiographic treatment with either glass or resin Y-90 microspheres.
Glass treatments were based on the partition model with a goal dose of 120 GY to the liver and calculated using the standard treatment window illustrator (BTG Biocompatibles Ltd). Resin treatments were based on the Body Surface Area (BSA) method and calculated using the SMAC (SIR-Spheres Microspheres Activity Calculator, Sirtex Medical). Both dosing calculations accounted for patient lung shunt fraction. In accordance with the Instructions for Use (IFU), all but four patients received treatment doses under 20% lung shunt fraction. For those four patients with greater than 20%, radiation dose to the lungs was calculated not to exceed a one-time dose of 25 Gray nor cumulative dose of 50 Gray. No extended shelf life treatments or treatments to extrahepatic arteries were performed. Following treatment, patients underwent follow-up CT or MR imaging at approximately one and six months to monitor tumor response.
Mean time-to-death and survival outcomes were compared between glass and resin treatment groups and by PVI for patients with HCC. Extent of PVI was documented for each patient, including segmental v. lobar and presence of extrahepatic disease. However, due to small sample sizes, patients with any amount of PVI at first treatment were classified as having PVI by the senior interventional radiologist for use in analyses. Time-to-death was defined as the number of days between initiation of Y-90 treatment and date of death due to any cause within 365 days. Participants known to be alive at the end of the follow-up period were censored at 365 days, while participants lost to follow-up were censored at the date of last contact. Survival was assessed by both treatment type and primary disease pathology. Overall survival was defined as the number of participants who were still alive at one year, divided by the total number of participants.
Participant and treatment characteristics were summarized using descriptive statistics, including mean and standard deviation for continuous variables and count and percentage data for categorical variables. Kaplan-Meier curves, log-rank tests, and Hazard Ratios (HRs) were used to compare and describe survival between treatment and primary pathology groups. Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC) and p-values of 0.05 were used to determine statistical significance for all calculations.
Results
A total of 114 patients were included in this study, with patients nearly evenly split between glass (51.8%, n=59) and resin (48.2%, n=55; (Table 1)) Y-90 treatments. Most patients were white (87.7%), non-Hispanic (98.2%), and male (61.4%), with a mean age at first Y-90 treatment of 60.8 years (SD ± 11.1). However, there were significantly more males who received glass (72.9%) than resin treatments (49.1%, p<0.01).
Table 1: Participant Demographic and Clinical Characteristics by Y-90 Treatment Type (n=114). * Patients may have had more than one liver disease or liver therapy prior to Y-90 Treatment 1; † Chi-square was used to test any PVI versus none and any prior liver therapy versus none; PVI = Portal Vein Invasion, AST = Aspartate Aminotransferase, AFP = Alpha-fetoprotein, ALT = Alanine Aminotransferase, ALP = Alkaline Phosphatase, CEA = Carcinoembryonic Antigen, MELD = Model for End-stage Liver Disease, TACE = Transcatheter Arterial Chemoembolization, MWA = Microwave Ablation |
The majority of patients (42.2%, n=48) had primary HCC pathology, while 28.9% (n=33) had mCRC of the liver, and 28.9% (n=33) had other metastatic disease to liver. There were no significant differences in the distribution of primary liver pathologies (p=0.47), types of liver disease (all p>0.05), number of lesions treated (0.75), median lesion size (0.81), percent of liver invaded by tumor (p=0.31), PVI at first Y-90 treatment (p=0.43), or median MELD scores (p=0.93) between treatment groups. Nearly one-quarter (22.8%) of patients did not receive any liver therapy prior to their first Y-90 treatment, while half (49.1%) had chemotherapy and 28.9% had other locoregional liver therapy. Overall, patients received their first Y-90 treatment a median of 44.5 days after baseline imaging (range=0 -166 days). Patients in both treatment groups had an average of 1.6 (SD ± 0.6) Y-90 treatments each, with the majority (54.4%) having two treatments (p=1.00; (Table 2)).
Table 2: Y-90 Treatment Course by Treatment Type (n=114). |
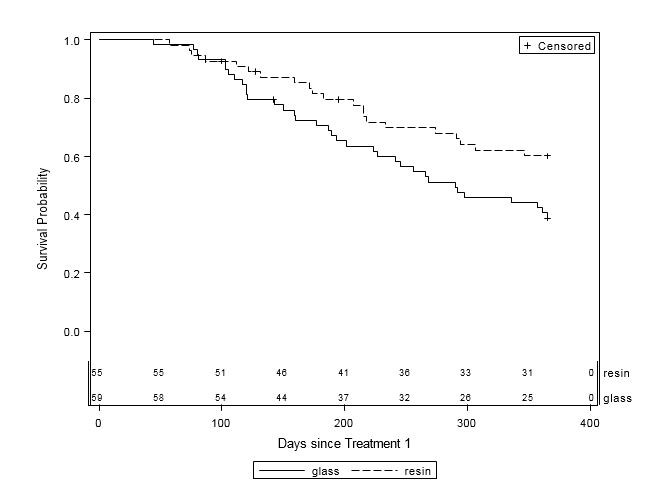
Overall, patients who received treatment with resin survived longer than those who received glass (Table 3, Figure 1). Nearly 60% of the 59 patients who received glass died within one year of their first treatment, while only 38.2% of the 55 patients who received resin died during the same timeframe (p=0.03). Among the remaining patients, one-third (37.3%) in the glass group and over half (56.4%) in the resin group were alive at one year, while 3.4% of glass patients and 5.5% of resin patients were lost to follow-up. Patients who received resin treatment were 44% less likely to die within one year compared to those who received glass treatment (HR=0.56, 95% CI=0.32-0.96, p=0.03). Estimated mean survival time for glass patients was 260.5 days (SE=14.5) compared to 283.7 days (SE=12.9) for resin patients.
Table 3: One-Year Survival by Y-90 Treatment Type (n=114). |
|||||||||||||||||||||||
|
Figure 1: One-Year Survival Curves by Y-90 Treatment Type (n=114). Glass (solid line, n=59) and resin (dashed line, n=55) treatments are displayed. The x-axis is survival time (in days) after first Y-90 treatment up to one year. The y-axis is the probability of survival to time x. Subjects were censored at date of last contact within 365 days of treatment or at 365 days, whichever occurred first. There was a significant difference in one-year survival between treatment groups (p=0.03), with an estimated 44% reduction in risk of death for patients in the resin treatment group compared to the glass group (HR=0.56, 95% CI=0.32-0.96). |
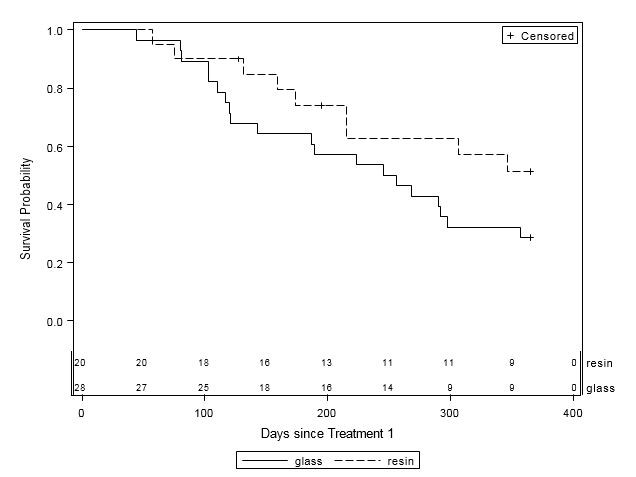
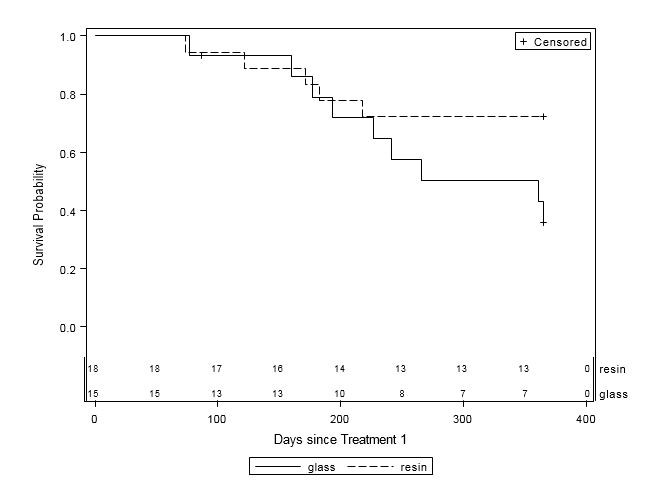
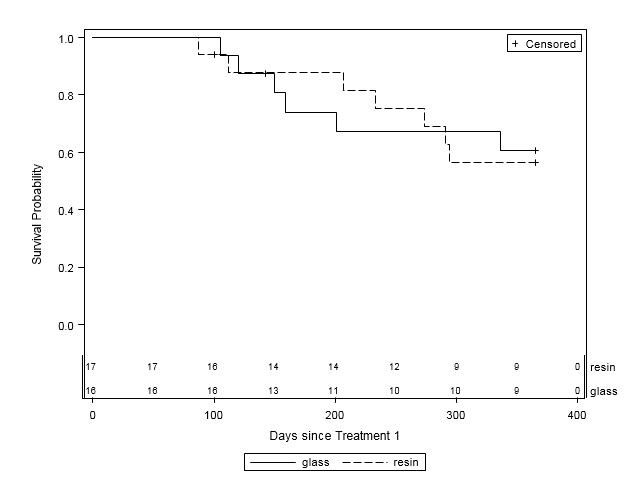
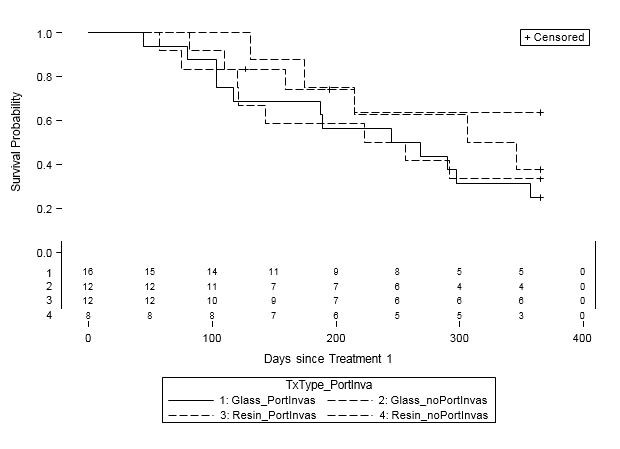
When examined by liver disease pathology (HCC, mCRC, and other), there were no statistically significant differences in one-year survival between treatment groups (Table 4, Figures 2A-C). For patients with HCC, estimated mean survival time was 270.4 days (SE=24.3) for resin treatments compared to 231.0 days (SE=21.2) for glass (HR=0.54, 95% CI=0.25-1.19). Moreover, when HCC patients (n=48) were further examined by presence of PVI, no statistically significant differences in one-year survival could be discerned, although estimated mean survival times ranged from 185.0 days (SE=18.7) to 276.3 days (SE=33.0; Table 5, Figure 3). For those with mCRC and other liver pathologies, patients treated with glass had longer estimated mean survival times than resin, although the difference was also not statistically significant (mCRC: HR=0.40, 95% CI=0.13-1.19; other pathologies: HR=1.07, 95% CI=0.36-3.18;).
Table 4: One-Year Survival by Y-90 Treatment Type and Primary Liver Pathology (n=114). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Figure 2A: One-Year Survival Curves for HCC Patients by Y-90 Treatment Type (n=48). Glass (solid line, n=28) and resin (dashed line, n=20) and treatments are displayed. For patients with HCC, there was no significant difference in one-year survival between treatment groups (HR=0.54, 95% CI=0.25-1.20). |
|
Figure 2B: One-Year Survival Curves for mCRC Patients by Y-90 Treatment Type (n=33). Glass (solid line, n=15) and resin (dashed line, n=18) treatments are displayed. For patients with mCRC, there was no significant difference in one-year survival between treatment groups (HR=0.40, 95% CI=0.13-1.20). |
|
Figure 2C: One-Year Survival Curves for Other Liver Pathology Patients by Y-90 Treatment Type (n=33). Glass (solid line, n=16) and resin (dashed line, n=17) and are displayed. For patients with other liver pathologies, there was no significant difference in one- year survival between treatment groups (HR=1.07, 95% CI=0.36-3.18). |
Table 5: One-Year Survival for HCC Patients by Y-90 Treatment Type and Portal Vein Invasion (PVI) Status (n=48). |
||||||||||||||||||||||||||||||||
|
Figure 3: One-Year Survival Curves for HCC Patients by Treatment Type and Portal Vein Invasion (PVI) Status (n=48). Glass treatments with PVI (solid line, n=16) and without PVI (short dashed line, n=12) and resin treatments with PVI (long and short dashed line, n=12) and without PVI (long dashed line, n=8) are displayed. For patients with HCC, there was no significant difference in one-year survival between treatment groups or PVI status. |
Finally, the most commonly reported complications of Y-90 treatment were fatigue (28.9%), abdominal pain (26.3%), and nausea and vomiting (14.9%) in both the resin and glass treatment groups (Table 6). There were no significant differences in reported complications by treatment group. Moreover, no patients experienced serious complications of radiation pneumonitis, biloma formation, cholecystitis, or intrahepatic abscess.
Table 6: Complications by Y-90 Treatment Type (n=114). |
Discussion
Overall, this study found that patients who received Y-90 treatment with resin microspheres had statistically significantly longer survival times compared to those treated with glass, with resin patients experiencing a 44% reduction in the likelihood of death within one year after initiation of Y-90 treatment. However, when examined by liver disease pathology and presence of PVI, no statistically significant differences could be determined for patients with HCC, mCRC, or other pathologies. The inability to detect statistically significant differences in these subgroups may be attributable to their relatively small sample size.
Although several prior studies support the efficacy of radioembolization in treating primary and secondary hepatic disease, few have compared survival outcomes for both types of Y-90 microsphere treatments [4,9-12,16]. Five studies were found to directly compare glass versus resin treatment, three comparing patients with HCC, one comparing patients with HCC and PVI and one comparing patients with mCRC [8,13,14,17,18]. A meta-analysis of HCC and mCRC liver metastases comparing Yttrium-90 microsphere types was also published by Vente et al., in 2009 [19]. The current study found a higher likelihood of survival across all patients treated with resin compared to glass. No other study has compared glass to resin treatments across all patients with HCC, mCRC and other hepatic metastases.
When specifically examined by liver disease pathology, the current study’s findings align with the 2017 study by Van der Gucht et al., which was unable to detect a statistically significant survival difference between HCC patients treated with glass (n=36) and resin (n=41) microspheres (p=0.77) [13]. In HCC patients with PVI specifically, a 2016 study by Biederman et al., reported a statistically significantly greater survival benefit attributable to glass (n=69) compared to resin (n=21) microspheres (p<0.001) [14]. The current study found a mean survival of 231.8 days in HCC patients with PVI treated with glass (n=16) compared to 185.0 days in resin treatment patients (n=12), though this was statistically insignificant, possibly due to a small sample size. Additionally, results of a study examining survival outcomes in mCRC patients have suggested a higher likelihood of overall survival for resin (n=13) compared to glass (n=15) treatments, but only in the second year after Y-90 treatment (p=0.0175) [8]. The meta-analysis published by Vente et al., demonstrated no significant difference between glass and resin treatment in mCRC patients though reported a significant benefit in HCC patients when treated with resin compared to glass [19]. The smaller sample size of the current study did not adequately power these subgroup comparisons by disease pathology or presence of PVI.
One possible explanation for why patients treated with resin mircrospheres may have longer survival times than patients treated with glass, may be related to the macroembolization effect of resin microspheres resulting in increased tumor ischemia. Not only are resin microspheres (20-60 µm) often larger than glass microspheres (20-30 µm) [6,7] but they also have a lower density of Y-90 per sphere than glass microspheres [6,7]. This translates into requiring more resin microspheres to administer a given dose, which can create a higher embolic effect with a given administration [20]. Complete dose delivery for glass microspheres is accomplished without detectable angiographic evidence of embolization, whereas, full delivery of resin microspheres is not always possible, as the target artery can develop stasis prior to completion of infusion [21]. Ultimately, these differences may result in increased tumor ischemia by resin microspheres rather than increased radiation dose.
This study is not without limitations. First, as a single institution retrospective study, the results are generalizabile only to similar patient populations and may result in selection bias from the inability to randomize patients to treatment types. The retrospective nature also reduces control over the availability of data for all subjects, such as ascites, encephalopathy, and performance status information. Second, although this study has a larger patient population than prior studies, small sample sizes within subgroup analyses by liver disease pathologies and PVI resulted in insufficient power to detect statistically significant results for these patient groups.
These small sample sizes also required us to combine all non-HCC and mCRC patients into a single ‘other pathology’ category, although many of the pathologies in this group clinically behaved differently. Larger prospective, randomized-controlled studies would improve the ability to better detect true significant differences in these patient groups and may be able to specifically explore the role of Y-90 treatments for certain types of secondary hepatic metastatic disease, such as neuroendocrine and breast metastases. Third, varying Y-90 treatment characteristics, such as differences in decisions between interventional radiologists regarding use of glass or resin microspheres, time between treatments, and unilobar versus bilobar treatments, may have resulted in differences that could have influenced survival outcomes. Finally, the overall survival time of this study was censored at 1 year, which makes comparison to other studies more difficult as many other studies report overall survival past one year.
Currently, glass microsphere radioembolization is FDA approved for treatment of primary HCC, while resin microsphere radioembolization is FDA approved only for treatment of mCRC [7]. Although the current study was unable to detect a significant difference in survival time by treatment type for differing liver pathologies, the results suggest Y-90 treatments may trend towards improved survival outcomes for patients with liver pathologies other than those for which they were originally approved. As a result, larger, prospective studies comparing tumor response and survival outcomes for glass and resin radioembolization treatment by specific disease pathologies are warranted. In sum, this study found a significant overall survival benefit of resin compared to glass Y-90 microsphere treatment, but was insufficiently powered to detect statistically significant survival differences by liver disease pathology, specifically HCC, PVI, mCRC, and other pathologies, between treatment groups.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Ibrahim SM, Nikolaidis P, Miller FH, Lewandowski RJ, Ryu RK, et al. (2009) Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom imaging 34: 566-581.
- Memon K, Lewandowski RJ, Kulik L, Riaz A, Mulcahy MF, et al. (2011) Radioembolization for primary and metastatic liver cancer. Semin Radiat Oncol 21: 294-302.
- Kennedy AS, Salem R (2010) Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer j 16: 163-175.
- Ibrahim SM, Lewandowski RJ, Sato KT, Gates VL, Kulik L, et al. (2008) Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol 14: 1664-1669.
- Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, et al. (2007) Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 68: 13-23.
- Kallini JR, Gabr A, Salem R, Lewandowski RJ (2016) Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Advances in therapy 33: 699-714.
- Srinivas SM, Nasr EC, Kunam VK, Bullen JA, Purysko AS (2016) Administered activity and outcomes of glass versus resin (90)Y microsphere radioembolization in patients with colorectal liver metastases. Journal of gastrointestinal oncology 7: 530-539.
- Raval M, Bande D, Pillai AK, Blaszkowsky LS, Ganguli S, et al. (2014) Yttrium-90 Radioembolization of Hepatic Metastases from Colorectal Cancer. Frontiers in oncology 4: 12-45.
- Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, et al. (2013) Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 57: 1826-1837.
- Hilgard P, Hamami M, Fouly AE, Scherag A, Muller S, et al. (2010) Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 52: 1741-1749.
- Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, et al. (2010) Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 138: 52-64.
- Van Der Gucht A, Jreige M, Denys A, Blanc-Durand P, Boubaker A, et al. (2017) Resin versus Glass Microspheres for Yttrium-90 Transarterial Radioembolization: Comparing Survival in Unresectable Hepatocellular Carcinoma using Pretreatment Partition Model Dosimetry. Journal of nuclear medicine 58: 1334-1340.
- Biederman DM, Titano JJ, Tabori NE, Pierobon ES, Alshebeeb K, et al. (2016) Outcomes of Radioembolization in the Treatment of Hepatocellular Carcinoma with Portal Vein Invasion: Resin versus Glass Microspheres. Journal of vascular and interventional radiology 27: 812-821.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, et al. (2009) Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Biomed Inform 42: 377-381.
- Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, et al. (2011) Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 54: 868-878.
- Rohr A, Haverkamp B, Pedersen W, Vavricek J, Iqbal S, et al. (2017) Retrospective Review of Tumor Response to Glass and Resin Y-90 Microsphere Treatments in Patients with Hepatocellular Carcinoma. Research Journal of Oncology 1: 8.
- Lam G, Seinstra B, van den Bosch M, Louie J, Sze D (2013) Comparison between resin and glass microspheres for Yttrium-90 radioembolization treatment of hepatocellular carcinoma. Journal of Vascular and Interventional Radiology 24: 149.
- Vente MA, Wondergem M, van der Tweel I, van den Bosch MA, Zonnenberg BA, et al. (2009) Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 19: 951-959.
- Piana PM, Bar V, Doyle L, Anne R, Sato T, et al. (2014) Early arterial stasis during resin-based yttrium-90 radioembolization: incidence and preliminary outcomes. HPB: the official journal of the International Hepato Pancreato Biliary Association 16: 336-341.
- Sato K, Lewandowski RJ, Bui JT, Omary R, Hunter RD, et al. (2006) Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovascular and interventional radiology 29: 522-529.
 LOGIN
LOGIN REGISTER
REGISTER.png)