Research Article
Anti-Diabetic Effects of Niclosamide Ethanolamine and Metformin in
Mouse Models
Hanlin Tao1, Jingjing Guo2, Amer Hassa Al-Asadi1, Shengkan Jin1*
1Department of Pharmacology, Rutgers University, Piscataway, New Jersey 08854, USA
2Department of Food Science, Rutgers University, New Brunswick, New Jersey 08901, USA
*Correspondence author: Shengkan Jin, Department of Pharmacology, Rutgers University-Robert Wood Johnson Medical School, 675 Hoes Lane West, R549, Piscataway, NJ 08854, USA, Tel: +1 7322354329, Fax: +1 7322354073; E-mail: jinsh@rwjms.rutgers.edu
Citation: Tao H, Guo J, Al-Asadi AH, Jin S (2017) Anti-Diabetic Effects of Niclosamide Ethanolamine and Metformin in Mouse Models. J Diabetes Endocrinol Metab Disord 2017: 1-8. doi:https://doi.org/10.29199/DEMD.101014
Received Date: 11 November 2017; Accepted Date: 15 December 2017; Published Date: 30 December 2017
Abstract
We compared the anti-diabetic effects of mitochondrial uncoupler Niclosamide Ethanolamine (NEN) and the first-line drug metformin, and examined potential combinatory effect or drug-drug interaction between the two compounds in mouse models. We treated the High-Fat Diet (HFD)-induced diabetic and the db/db mice with NEN (~200 mg/kg.day), metformin (~75 mg/kg.day), or the two compounds together through oral administration, and monitored the exposure of the two compounds and effect on improving diabetic conditions. At the dosages tested in the present study, the co-treatment leads to plasma and liver exposures of NEN and metformin that are similar to treatment with each drug alone. In both animal models, NEN alone is more effective than metformin alone in reducing blood glucose. The co-treatment is significantly better than metformin alone in lowering blood glucose. NEN treatment alone is effective in reducing hepatic lipid accumulation, while metformin treatment does not. Moreover, the co-treatment appears to have a better effect in reducing hepatic steatosis and liver damage than NEN treatment alone. Our data support that NEN and metformin co-treatment does not compromise the hypoglycemic effect of metformin, and the co-treatment may have additional benefit of improving hepatic steatosis.
Keywords: Insulin Resistance; Metformin; Mitochondrial Uncoupling; Niclosamide; Type 2 Diabetes
Abbreviations
ALT : Alanine Aminotransferase
AST : Aspartate Aminotransferase
HFD : High-Fat Diet
MET : Metformin
NEN : Niclosamide Ethanolamine
Introduction
Type 2 diabetes has become one of the most challenging health problems in the United States and around the world [1,2]. Currently, metformin is the first-line drug for treating type 2 diabetes. The function of metformin has been attributed to its suppression of hepatic gluconeogenesis, though the underlying mechanism remains unclear. It was initially believed that metformin is a mitochondrial respiratory chain complex I inhibitor, through which metformin indirectly inhibits hepatic gluconeogenesis [3,4]. Later studies support that metformin activates AMP-Activated Protein Kinase (AMPK) and leads to reduction of gluconeogenic gene transcription [5-7]. More recently, metformin is found to inhibit the mitochondrial glycerophosphate dehydrogenase directly, thereby reduces conversion of lactate and glycerol to glucose and results in decrease in hepatic gluconeogenesis [8].
Although metformin is effective in lowering blood glucose without posing a risk of hypoglycemia, patients often become refractory to the therapy [9]. We have recently demonstrated that niclosamide (5-chloro-salicyl-(2-chloro-4-nitro) anilide) ethanolamine (NEN), which induces mild mitochondrial respiration uncoupling, can effectively improve hepatic insulin sensitivity and decrease blood glucose in diabetic mouse models [10]. Niclosamide is an anthelmintic drug approved by the US Food and Drug Administration (FDA) for treating intestinal infection of tapeworms [11,12] and NEN has an excellent safety profile in mammalian animals [13,14]. Given the excellent safety profile, NEN and the mechanism of action NEN may represent a promising novel therapeutic strategy for treating insulin resistance and type 2 diabetes. In our previous study, we have demonstrated that NEN reduces insulin resistance in diabetic mice by stimulating oxidation of fatty acid and reducing hepatic lipid accumulation, which represent a different mechanism of action from that of metformin. As metformin is the first line medication for treating type 2 diabetes, in the present study, we examined the efficacy of a combinatory treatment with NEN and metformin in treating type 2 diabetes in mouse models, and determined if NEN might interfere with the action of metformin.
Materials and Methods
Reagents and materials
Niclosamide ethanolamine salt (NEN, niclosamide 5-chloro-salicyl-(2-chloro-4-nitro) anilide 2-aminoethanol salt) from 2A PharmaChem (Lisle, IL); Metformin hydrochloride from BOC Sciences (Shirley, NY); Insulin (Humulin-R Insulin U-100) from Eli Lilly (Indianapolis, IN).
Animals and treatments
Diet-Induced Obesity (DIO) C57BL/6J mice (Stock# 380050) and db/db mice (BKS.Cg-Dock7m +/+ Leprdb/J, Stock# 000642) were from the Jackson Laboratory (Bar Harbor, ME). Experimental protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at the Rutgers University. For the DIO C57BL/6J mice, 6-week old animals were fed on high-fat diet (HFD, 60% fat calorie) for three months to induce obesity and diabetes. The mice were then either maintained on HFD, or switched to HFD containing 2,000 p.p.m. NEN, or HFD and 75 mg/kg.day metformin in drinking water, or HFD containing NEN and metformin in drinking water. For the db/db mice, 5-week old animals were fed on either normal rodent diet AIN-93M, or AIN-93M diet containing 2,000 p.p.m. NEN, or AIN-93M and 75 mg/kg.day metformin in drinking water, or AIN-93M containing NEN and metformin in drinking water. Mouse water intake, food intake and body weight were monitored every week. The NEN dose is around 200 mg/kg.day according to the average food intake and body weight; the concentration of metformin was adjusted by the amount of average daily water intake and body weight to make sure the dose of metformin as indicated in the experiments. All the mouse diets are prepared by the Research Diet, Inc. (New Brunswick, NJ).
Analyses of plasma and hepatic NEN and metformin
Plasma and hepatic NEN and metformin levels were determined using LC/MS/MS method by WuXi Apptech, Inc. (Plainsboro, NJ) using method previously described with minor modification [15].
Briefly, NEN was mixed into the diet and metformin was dissolved into the drinking water, the concentrations were determined by the daily food and water intake per mouse. The mice were treated for 2 weeks to let the animals completely accommodated to the diet and drinking water.
Blood samples were collected through a nick on lateral tail vein at indicated time points, and 10 µl plasma was mixed with 2 µl methanol and 200 µl of 200 ng·ml-1 internal standard in acetonitrile, kept at -20o for 30 min, 10 µl of supernatant after vortex and centrifuge subjected to LC/MS/MS analyses.
For tissue distribution studies, 100 mg of liver tissue was homogenized with 5X volume of water (500 µl), 50 µl of the homogenate was mixed with 5 µl methanol and 300 µl of 200 ng·ml-1internal standard in acetonitrile, kept at -20o for 30 minutes, and then vortex for 1 min and centrifuge at 12,000 g for 15 min. 10 µl of supernatant was subjected to LC/MS/MS analyses.
Blood glucose, insulin sensitivity assay and plasma insulin assay
The mice for measuring blood glucose were fast overnight, and then blood glucose concentrations were determined using the OneTouch UltraSmart blood glucose monitoring system (Lifescan).
For measuring plasma insulin, the mice were fasted for 5-6 h. The insulin concentrations in the plasma samples were analyzed by the US National Mouse Metabolic Phenotyping Center (MMPC) at University of California, Davis (UC Davis).
For insulin sensitivity assay, the mice were first fasted for 5-6 h, and then intraperitoneally injected with insulin at a dose of 0.75 unit·kg-1 of body weight. The blood glucose concentrations were then measured at time points 0, 15, 30, 60, 90, and 120 min after the insulin injection.
Mouse liver histological analyses and lipid profile analyses
For liver histological studies, mouse liver tissues were fixed with neutral buffered formalin 10% (Surgipath Medical Industries, Inc.) and embedded in paraffin. Tissue sections were prepared and stained with Hematoxylin and Eosin (H&E). The liver tissues conserved by flash freezing in liquid nitrogen were used for hepatic total TriGlycerides (TG) and Total Cholesterol (TC) determination (MMPC at UC Davis).
Statistical analysis
Statistical significance (p) was determined by one-way ANOVA, or Student’s t test. All error bars represent s.d.; Data are presented as means ± s.d. and statistical significances are denoted as indicated.
Results
Determining plasma and hepatic NEN and metformin levels in mice
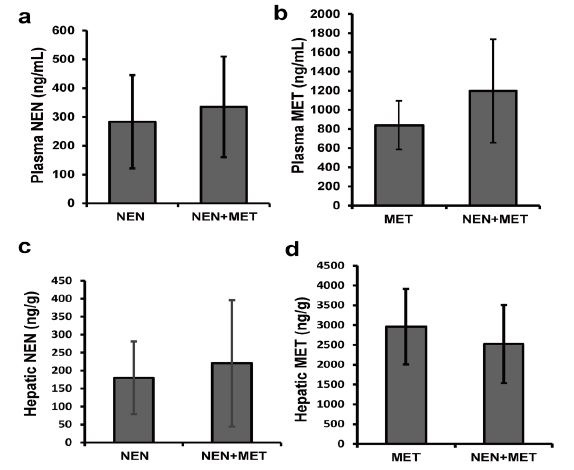
We first compared the exposure of NEN and metformin in mice treated with NEN alone, metformin alone, and NEN plus metformin. 3 groups of C57BL/6J mice were treated orally with NEN alone (~200 mg/kg.day, mixed with diet), metformin alone (~75 mg/kg.day, dissolved in drinking water), or NEN plus metformin for 2 weeks. The concentrations of each drug in plasma and liver of the treated mice were then analyzed under fed condition. The mice had free access to diet and drinking water in all day time. We collected plasma and liver samples at different time points (9:00 p.m., 9:00 a.m., 12:00 p.m., 15:00 p.m., and 18:00 p.m.) to determine the fluctuation of drug concentrations during a whole-day cycle. Our result showed that the levels of NEN and metformin in plasma and liver do not fluctuate significantly over a period of 24 hours when mice had been accommodated to the diet and water (data not shown). In mice treated with NEN alone, we detected an average of 283 ng/ml NEN in plasma (Figure 1a) and an average of 180 ng/g NEN in liver (Figure 1c). In mice treated with metformin alone, we detected an average of 839 ng/ml metformin in plasma (Figure 1b), which is with in the range of the documented steady-state metformin plasma concentrations in human patients [16]. The average hepatic metformin level was 2,964±954 ng/g (Figure 1d). In mice treated with both drugs, we detected the average concentration of NEN at 335±175 ng/ml in plasma (Figure 1a) and 220±176 ng/g in liver (Figure 1c); and the average levels of metformin are 1,198±540 ng/ml in blood (Figure 1b) and 2,523±985 ng/g in liver (Figure 1d). No significant difference in the plasma or hepatic concentration was observed for either drug between mice treated with either drug alone and mice treated with both drugs. We concluded that exposure of NEN (or metformin) is not affected by the co-administration with metformin (or NEN).
|
Figure 1: Pharmacokinetic study of NEN and metformin in mice. Plasma concentration of (a) NEN and (b) metformin; hepatic concentration of (c) NEN and (d) metformin from mice which were administrated with NEN (~200 mg/kg.day, NEN), or metformin (~75 mg/kg.day, MET), or both NEN and metformin (NEN+MET) for 2 weeks. n = 3. |
Effect of NEN and metformin in reducing blood glucose and improving insulin sensitivity in HFD-induced diabetic mice
We then compared the glucose lowering effect of NEN and metformin in HFD-induced diabetic mice. Starting at 6-week of age, 32 male C57BL/6J mice were fed on HFD for 3 months to induce insulin resistance and hyperglycemia. The mice were then randomized into 4 groups and fed HFD only (Control group), HFD containing NEN (~200 mg/kg.day, NEN group), HFD containing metformin (~75 mg/kg.day, MET group), or HFD containing NEN and metformin (NEN+MET group), respectively. After 2 weeks of treatment, the blood glucose levels in the mice were measured. In all three treatment groups, the fasting blood glucose levels were significantly reduced as compared to that of control mice. As shown in figure 2a, the blood glucose levels were decreased from an average of 132 mg/dl to 94 mg/dl (reduction of 28.8%) in NEN group, from an average of 130 mg/dl to 114 mg/dl (reduction of 12.3%) in MET group, and from an average of 127 mg/dl to 81 mg/dl (reduction of 36.2%) in the combinatory treatment group. At the dosage tested in this study, the combinatory treatment led to a more dramatic blood glucose decrease, which are significantly lower compared to the treatment with MET alone group, but not significant compared to the treatment with NEN alone group. Therefore, it seems that NEN and MET do not have synergistic effect in terms of lowering blood glucose, nor do they interfere with each other.
We further examined insulin sensitivity and plasma insulin levels in the mice. The mice in control and MET groups exhibited higher plasma insulin levels than those in NEN or combinatory treatment groups (Figure 2b), reflecting a compensatory increase of insulin secretion in response to insulin resistance. Consistently, the mice in NEN and combinatory treatment groups exhibited improved insulin sensitivity compared to the mice in the control group in insulin tolerance assay, while the mice in MET group showed a similar level of insulin sensitivity as the control mice (Figure 2c). These data suggested at the dosages of NEN and metformin used in this study, NEN treatment significantly improved the insulin sensitivity of the diabetic mice, while metformin did not. HFD feeding caused body weight increase in the control mice. NEN treatment significantly slowed down the increase of body weight, which can also be found in the combinatory treatment group but not in the metformin treatment group (Figure 2d). NEN treatment slightly increased mouse food intake, but was not significant compared to the other three groups (Figure 2e).
|
Figure 2: Effect of NEN and metformin on glycemic control and insulin sensitivity in HFD-induced diabetic mice. 5-week-old mice were fed on HFD for 3 months, and then either continued to feed on HFD (Control), or switched to HFD containing NEN (NEN), HFD and Metformin (MET), or HFD plus NEN and metformin (NEN+MET) for another 6 weeks. (a) Fast blood glucose before and after 2 weeks of drug treatment, (b) plasma insulin and (c) insulin sensitivity assay at 4 weeks of drug treatment, (d) body weight measured before and after 5 weeks of drug treatment, and (e) daily food intake. n = 8. * P <0.05, ** P < 0.01, and *** P < 0.001 versus Control; # P < 0.05, ## P < 0.01, ### P < 0.001 versus MET. |
Effects of NEN and metformin in controlling hepatic steatosis and liver damage in mice
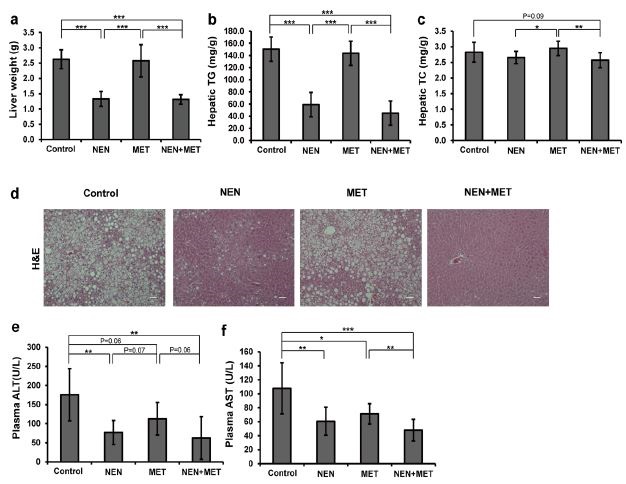
We had previously demonstrated that NEN improves insulin sensitivity in diabetic mice through depletion of ectopic lipid accumulation in mouse liver. Here we examined the hepatic content of lipid accumulation in the mice to compare the effect of metformin or the combinatory treatment. After fed HFD for 3 months, mice developed severe hepatic steatosis, as shown by liver weight measurement, and by histological analyses and lipid content quantification in the control mice (Figure 3a-d). NEN treatment dramatically reduced liver weight (Figure 3a) and triglyceride accumulation in liver (Figure 3b and d), even though the mice were continuously fed HFD. The levels of lipid accumulation in liver in mice of the combinatory treatment group tended to be even lower when compared to those in mice treated with NEN alone (Figure 3b-d). However, treatment with metformin alone did not have any effect in reducing liver fat accumulation.
Hepatic steatosis may cause liver damage leading to inflammation and scarring in liver. Plasma Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) levels are usually elevated along with development of hepatic steatosis, indicating certain degree of liver damage. To investigate whether the depletion of hepatic lipid accumulation induced by NEN can protect the liver, we analyzed plasma ALT and AST levels in the mice. Consistently, we found an average ALT level at 175.8 U/L (Figure 3e) and an average AST level at 107.8 U/L (Figure 3f) in the control mice after long term HFD feeding. We found that at the testing dosage, metformin treatment marginally reduced the average ALT and AST levels to 112.5 U/L (p = 0.06) and 71.2 U/L (p = 0.05), while NEN treatment significantly reduced the average ALT level to 76.8 U/L and the average AST level to 60.6 U/L (p < 0.01 for both, compared to the controls). And the combinatory treatment caused a more dramatic reduction in plasma ALT and AST levels to 62.7 U/L (p < 0.01) and 47.7 U/L (p < 0.005), which are almost comparable to the levels of healthy mice [17].
|
Figure 3: Effect of NEN and metformin on hepatic steatosis and liver damage in HFD-induced diabetic mice. (a) Liver weight, (b) hepatic Triglycerides content (TG), (c) hepatic Total Cholesterol content (TC), and (d) representative images of H&E stained liver tissues (scale bar = 50μm), (e) plasma ALT and (f) plasma AST levels. Mice were fed HFD (Control), HFD containing NEN (NEN), HFD and Metformin (MET), or HFD containing NEN and Metformin (NEN+MET) for 6 weeks after initial 3-month HFD feeding. n = 8. * P < 0.05, ** P < 0.01, and *** P < 0.001. |
Effect of NEN and metformin in db/db mice
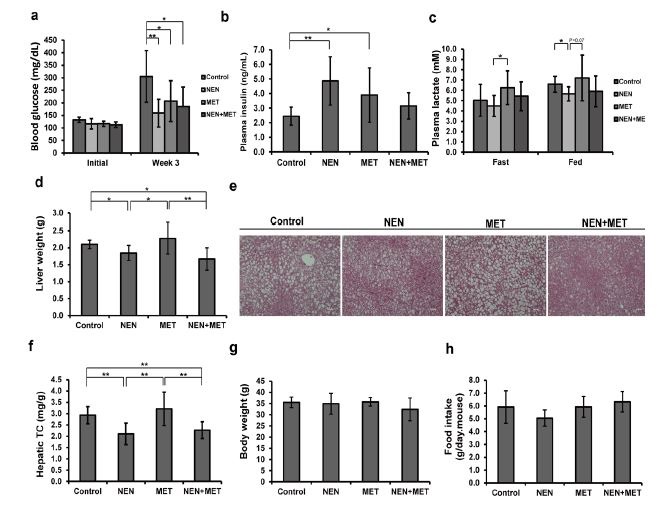
We further examined the anti-diabetic effect of NEN and metformin in the db/db mice, a classic genetic model of type 2 diabetes. The db/db mice develop severe hyperglycemic symptoms within 8 weeks of age, as a result of a genetic mutation in the leptin receptor gene. At the age of 5 weeks, we randomized db/db mice into four groups, and fed them standard mouse diet, or treated with NEN, metformin, or NEN and metformin respectively using the same dosages as described earlier. After three-week treatment, the fasting blood glucose levels were measured. Compared to high blood glucose (313.3 mg/dl) in the control mice, the NEN- treated mice exhibited a significantly lower average blood glucose level (159.1 mg/dl) (Figure 4a). The average blood glucose level was also lower in metformin treated mice (207.1 mg/dl), and in the combinatory treatment mice (184.9 mg/dl) (Figure 4a). Consistent with our previous report, plasma insulin concentrations declined rapidly in the db/db mice as the disease progressed [10]: in young db/db mouse the plasma insulin levels were usually higher than 5 ng/ml, and it dropped to around 2 ng/ml within the 4-week time of experiment in the control mice. The course of plasma insulin decline was slowed down significantly in the three drug-treated groups of mice (Figure 4b). Additionally, we measured blood lactate concentrations in the mice. Metformin treatment has been associated with an important adverse effect, the lactic acidosis [18]. By promoting the oxidation of pyruvate, NEN treatment reduced blood lactate concentration [10]. As shown in figure 4c, the NEN-treated mice exhibited lower levels of blood lactate as compared to the control or MET-treated mice; and the combinatory treatment also led to lower blood lactate levels when compared to the MET-treated mice. Therefore, a combinatory application of NEN and metformin may elicit beneficial effect by reducing the adverse effect of metformin on lactate production.
We further investigated the hepatic lipid accumulation in the db/db mice. Both liver weight measurement and histological analyses showed that a massive steatosis was developed in the liver of the control and MET groups of mice, whereas a lower liver mass and fewer lipid droplets were identified in the liver sections of mice under NEN and the combinatory treatment (Figure 4d and e), though the difference was not statistically significant in quantification of the triglycerides content (data not shown). However, the hepatic cholesterol levels in the mice treated with NEN alone or combinatory treatment were significantly lower than control group (Figure 4f). Again, there was no significant difference in food intake between the control and the treatment groups of mice (Figure 4h). The average body weight in all the groups showed no difference as well (Figure 4g).
|
Figure 4: Effect of NEN and metformin on diabetic symptoms in db/db mice. (a) Overnight fasting blood glucose measured at the indicated time points, (b) plasma insulin and (c) plasma lactate levels; (d) Liver weight, (e) representative images of H&E stained liver tissues (scale bar = 50μm), (f) hepatic total cholesterol content, (f) body weight, (h) daily food intake. The db/db mice fed standard mouse diet (Control), or treated with NEN (NEN), Metformin (MET), or NEN in combination with Metformin (NEN+MET) for 5 weeks. n = 8. * P < 0.05, ** P < 0.01, and *** P < 0.001. |
Discussion
Insulin resistance, which is closely associated with obesity and ectopic accumulation of fat in liver and muscles, precedes the development of type 2 diabetes [19,20]. Mild futile energy consumption induced by safe mitochondrial uncoupler, such as NEN, has been shown to reduce hepatic lipid accumulation and improve insulin sensitivity in animal models, and represents a promising new therapeutic strategy for treating type 2 diabetes [10]. Currently, metformin is widely used as the first line pharmacotherapy for treating type 2 diabetes. It is effective in reducing hepatic glucose production though mechanisms of action still inconclusive. It is critically important to address experimentally the potential drug-drug interaction between the mechanisms represented by NEN and by metformin, as in future clinical trials most type 2 diabetes patients would have been on metformin medication.
In the present study, we firstly examined the potential drug-drug interference in HFD-induced diabetic mice and db/db mice with treatment with either NEN or metformin or the combination of the two compounds. Our data firstly shows that the co-treatment does not alter the exposure of NEN or metformin as compared to each was administered alone, which suggests co-administration of one drug does not significantly affect the absorption, metabolism and excretion of the other drug. In addition, we did not observe any drug-drug interaction in the blood glucose lowering effect between NEN and metformin in the animal studies. When compared to the NEN treatment alone, a combinatory application of NEN and metformin elicit a slight improvement in reducing blood glucose level, hepatic fat accumulation, as well as liver damage as indicated by decrease of plasma ALT and AST levels. These data suggest that the mechanism of mild mitochondrial uncoupling induced by NEN does not interfere with the mechanism of action of metformin, which works mainly through inhibition of hepatic production of glucose. Mitochondrial uncoupling increases the consumption of acetyl-coA and intermediates of the Tricarboxylic Acid (TCA) cycle, which are potential substrates for hepatic gluconeogenesis; therefore, it is possible that NEN treatment alone might also have an impact on hepatic glucose production. This might explain why the combinatory treatment does not exhibit a synergistic effect but only a slight additional effect.
Consistent with our previous report, NEN is very effective in reducing hepatic fat accumulation and exhibits excellent activity of controlling blood glucose in the diabetic animals. At the dosages tested in the present study, NEN is much more effective in treating insulin resistance and hyperglycemia than metformin. In addition, oral NEN almost completely stopped the body weight gain in the mice which were still feeding the HFD, even though the food intakes of these NEN treated mice were slightly increased. Another recently published study reported that niclosamide has an anti-obesity effect in HFD-fed mice [21]. In the study, the free base niclosamide was used and a slight reduction in food intakes was noticed in the mice. In previous studies, metformin was also found to cause reduction of body weight gain and HFD intake in HFD-fed mice [22], however metformin were used at very high dosages (150, 300 mg/kg, p.o.). In patients with type 2 diabetes and obesity, oral metformin induces small weight losses at the beginning of the treatment [23], but the patients gradually regain the lost weight over the 10-year follow-up period [24]. The divergence of these observations might be related to the different dosages used in the studies, or the intrinsic difference between human and mouse models.
Clinical pharmacokinetic studies show that the therapeutic levels of metformin in patients are within 500 to 2,000 ng/ml range [25,26]. Some previous animal studies have used metformin doses ranging from 250 mg/kg.day to 500 mg/kg.day, leading to plasma metformin concentrations higher than 1 mM (>100,000 ng/ml) [20], which can never be achieved in clinical therapies. A dosage of 75 mg/kg.day, which gives the plasma and hepatic metformin concentrations within the therapeutic ranges observed in human patients, represents a physiologically and pharmacologically relevant condition [27,28]. At this dose, we found that metformin is effective in reducing blood glucose, but does not decrease hepatic lipid content or body weight. Some previous studies indicated metformin ameliorates hepatic steatosis in mice [29,30], but clinical data showed metformin does not affect liver fat content [31,32]. Again, the divergence of the observations in animal studies and in patients might be related to the intrinsic difference between human and mouse models.
In summary, we showed in mice that oral NEN administration does not interfere with the treatment with metformin. Based on the animal studies in this report, it is unlikely that the combinatory treatment will cause drug-drug interaction in patients due to the pharmacokinetic interaction or the mechanism of actions between the two compounds. Moreover, the combinatory treatment may result in potential additional beneficiary effects in improving blood glucose, improving hepatosteatosis, and reducing liver damage in patients.
Acknowledgements
The study was supported by funding from Mito BioPharm, LLC.NIH (R21 CA216604). We thank Dr. James Graham at the MMPC of University of California, Davis for multiple mouse metabolic phenotype characterization works; we also thank the Tissue Analytical Services (Lei Cong, Lucy Franciosa and Shafiq Bhat) at the Cancer Institute of New Jersey (CINJ) for assistance with histology.
References
- World Health Organization (WHO) (2016) Diabetes Fact sheet, WHO, Geneva, Switzerland.
- Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, et al. (2001) Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34: 1158-1163.
- Owen MR, Doran E, Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607-614.
- El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X, et al. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275: 223-228.
- Zhou G, Myers R, Li Y, Chen Y, Shen X, et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167-1174.
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420-2425.
- Meng S, Cao J, He Q, Xiong L, Chang E, et al. (2015) Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Bio Chem 290: 3793-3802.
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, et al. (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542-546.
- Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P, et al. (2012) Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med 156: 218-231.
- Tao HL, Zhang Y, Zeng XG, Shulman GI, Jin SK (2014) Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med 20: 1263-1269.
- Frayha GJ, Smyth JD, Gobert JG, Savel J (1997) The mechanisms of action of antiprotozoal and anthelmintic drugs in man. Gen Pharmacol 28: 273-299.
- Sheth UK (1975) Mechanisms of anthelmintic action. Prog Drug Res 19: 147-157.
- Andrews P, Thyssen J, Lorke D (1982) The biology and toxicology of molluscicides, Bayluscide. Pharmacol Ther 19: 245-295.
- National Library of Medicine (NLM) (2002) NICLOSAMIDE - National Library of Medicine HSDB, NLM, Maryland, USA.
- Chang YW, Teng-Kuang Y, Ke-Ta L, Wei-Cheng C, Hsien-Tsung Y, et al. (2006) Pharmacokinetics of anti-SARS-CoV agent niclosamide and its analogs in rats. J Food Drug Anal 14: 329-333.
- Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, et al. (2011) The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics 21: 837-850.
- Fernandez I, Pena A, Del Teso N, Perez V, Rodriguez-Cuesta J (2010) Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49: 202-206.
- Lalau JD (2010) Lactic Acidosis Induced by Metformin Incidence, Management and Prevention. Drug Saf 33: 727-740.
- Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444: 840-846.
- Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375: 2267-2277.
- Al-Gareeb AI, Aljubory KD, Alkuraishy HM (2017) Niclosamide as an anti-obesity drug: an experimental study. Eat Weight Disord 22: 339-344.
- Matsui Y, Hirasawa Y, Sugiura T, Toyoshi T, Kyuki K, et al. (2010) Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J mice. Biol Pharm Bull 33: 963-970.
- Golay A (2008) Metformin and body weight. Int J Obes (Lond) 32: 61-72.
- Diabetes Prevention Program Research G, Knowler WC, Fowler SE, Hamman RF, Christophi CA, et al. (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374: 1677-1686.
- Scheen AJ (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30: 359-371.
- Sum CF, Webster JM, Johnson AB, Catalano C, Cooper BG, et al. (1992) The effect of intravenous metformin on glucose metabolism during hyperglycaemia in type 2 diabetes. Diabet Med 9: 61-65.
- Wilcock C, Bailey CJ (1994) Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24: 49-57.
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, et al. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355-2369.
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, et al. (2000) Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6: 998-1003.
- Woo SL, Xu H, Li H, Zhao Y, Hu X, et al. (2014) Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS One 9: 91111.
- Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, et al. (2004) Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169-2176.
- Li Y, Liu L, Wang B, Wang J, Chen D (2013) Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep 1: 57-64.
 LOGIN
LOGIN REGISTER
REGISTER.png)