
Review Article
Fat and Lipid Partitioning: Phenotyping Beyond BMI
Guglielmi V1,2*, Morretti T2, Morazzini M2 and Sbraccia P1,2
1Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
2Internal Medicine Unit and Obesity Center, University Hospital Policlinico Tor Vergata, Rome, Italy
*Corresponding author: Guglielmi V, Department of Systems Medicine, University of Rome Tor Vergata, Via Montpellier 1, 00133 Rome, Italy, Tel: +39 3478615670; Fax: +39 0672596890; E-mail: valeria.guglielmi@uniroma2.it
Citation: Guglielmi V, Morretti T, Morazzini M, Sbraccia P (2017) Fat and Lipid Partitioning: Phenotyping Beyond BMI. J Diabetes Endocrinol Metab Disord 2017: 1-6. doi:https://doi.org/10.29199/DEMD.101011
Received: 07 July, 2017; Accepted: 17 July, 2017; Published: 03 August, 2017
Abstract
The world-wide obesity epidemic, predisposing towards several chronic diseases, not only poses a major public health issue, but also has a negative impact on individual’ life expectancy and quality. These two dimensions may need distinct diagnostic and preventive approaches. Indeed, whether BMI and total adiposity are positively correlated with cardiometabolic disease risk at the population level, adipose tissue regional distribution and lipid storage in ectopic tissues define the obesity phenotype and, thus, better predict the risk of developing obesity complications at the individual level.
Indeed, together with the more extensively studied intraabdominal adipose tissue, smaller ectopic visceral depots have, in the recent years, gained increasing attention for their putative role in mediating the local detrimental effects of obesity. It has also been increasingly appreciated that intracellular lipid accumulation in non-adipose tissues leads to pathological responses and impaired insulin signaling. This narrative review focuses on the phenotypic differences between different adipose depots that link their depot-specific biology to obesity specific complications.
Introduction
Owing to the modern sedentary lifestyle in both developed and developing countries, the prevalence of obesity has reached an alarming level and has become a worldwide epidemic. This has stimulated immense research interest on the pathophysiological role of adipose tissue in obesity-related complications and, thereby, gradually transformed adipose tissue from being considered a merely inert store for excess lipids into a metabolically active endocrine organ that secretes a wide range of potently bioactive signaling molecules with autocrine, paracrine and endocrine functions, and that is actively involved in metabolic homeostasis, insulin sensitivity and inflammation [1-3]. Moreover, White Adipose Tissue (WAT) has emerged as highly heterogeneous, each anatomical depot differing in intrinsic properties and phenotypic profiles. As such, the differential accumulation of fat in specific depots translates into different clinical outcomes.
Is it time to move beyond BMI: Body fat mass and distribution
Obesity is defined as an excess accumulation of body fat, and Body Mass Index (BMI) is the cornerstone of the current classification system for obesity and its advantages are widely exploited in both international surveillance and individual patient assessment. However, BMI like all anthropometric measurements, although inexpensive and simple to perform, are surrogate measures of body fatness and, especially in some clinical conditions [4], provide misleading information about body fat content. Besides, the use of universal BMI cut-off points to classify subjects as normal weight, overweight and obese, do not consistently reflect adiposity in different ethnic populations [5].
The adipose organ includes numerous anatomical depots. Major depots reside in Subcutaneous (SC) abdominal and gluteo-femoral region which represent >80% of total body fat, whereas intraperitoneal depots (also known as visceral adipose tissues), including the omental, the mesenteric and epiploic depots, represent the remaining 10~20% of total body fat in men and 5~10% in women. There are also numerous smaller visceral adipose depots that may serve specialized functions related to their neighboring tissues.
If total adiposity is positively correlated with cardiometabolic disease risk at the population level, on the other hand, regional fat distribution better predicts insulin resistance and related complications at the individual level [4].
Indeed, it is well established that accumulation of adipose tissue in the upper body (abdominal region) is associated with the development of obesity-related metabolic and cardiovascular complications and even all-cause mortality, whereas preferential fat accumulation in the lower body (gluteo-femoral region) is associated with a lower risk after adjustment for total body fat mass [6,7] and may be even protective [8].
This is evident in the case of partial lipodystrophies, disorders characterized by severe metabolic abnormalities associated with loss of SC fat [9,10], and thiazolidinediones, whose effects include expansion of SC adipose tissue resulting in improved cardiometabolic health [11]. Moreover, while omentectomy (in addition to bariatric surgery procedures) did not further improve the metabolic and inflammatory markers [11] in most of the studies and the effects of removal of abdominal SC fat via liposuction are inconsistent [12], instead femoral lipectomy worsened the lipid profile in a recent randomized trial [13], corroborating the clinical evidence that gluteo-femoral fat distribution should be regarded as beneficial [14].
The mechanisms that underlie inter-individual differences in body fat distribution are complex and remain to be elucidated although evidence indicates that sex hormones [15], use of glucocorticoids [16], genetic substrate [17] as well as epigenetic mechanisms [18,19] determine where the surplus energy is stored as fat. Also, ageing causes a redistribution of adiposity from SC to visceral compartments irrespective of gender and race [20]. Finally, genetics as well as environmental factors may contribute to ethnic differences in fat distribution [21,22].
Differences in the phenotype of upper and lower body obesity: Unraveling the underlying mechanisms
WAT has been gradually emerged to be a highly heterogeneous organ. Depot-specific differences are present in many species, suggesting an evolutionary advantage. This apparent compartmentalization of WAT and its inter- and intradepot heterogeneity seems to root from the diversity in developmental origin of adipocyte precursors and is gradually amplified during WAT expansion by intrinsic differences in adipocyte turnover, lipid metabolism and adipokine secretion profile [23].
Importantly, the inability to expand adipose tissue mass through adipocyte hyperplasia will evoke adipocyte hypertrophy during a prolonged positive energy balance. It is well established that enlargement of adipocytes is a key feature of dysfunctional adipose tissue [24]. Indeed, hypertrophic adipocytes have an impaired capacity of further storing dietary lipids, which results in a redirection of lipids towards other metabolic organs.
Compared to visceral depots, SC fat contains a larger pool of preadipocytes [25], which even display higher levels of adipogenic genes expression and respond better than those from visceral depots to differentiation cues [25], possibly according to an evolutionary conserved adipogenic program [26], also involving gene regulation by miRNAs, whose expression pattern is also dependent on fat depot [27]. As such, fat accumulation in visceral depots predominantly results from adipocyte hypertrophy while SC expands through hyperplasia [28]. This can be also partly explained by the fact that adipocytes and their precursors from visceral depots are respectively proner to death and more resistant to differentiation compared to those from SC. In only apparent contrast, it has been shown that, in mice, during diet-induced obesity development, adipogenesis increases in both SC and visceral depots of young animals but only in visceral depot of adults. Taken together, this data might suggest that, at the worsening of obesity, the obesity-related metabolic derangements are primarily due to failure of SC fat adipogenesis and plasticity [29]. In this view, SC fat not appropriately expanding to store the energy surplus, and in turn leading to ectopic fat deposition in other tissues involved in metabolic homeostasis (i.e., skeletal muscle, the liver, and visceral adipose tissue) and insulin resistance [30] would be a critical factor in the development of insulin resistance (Figure 1).
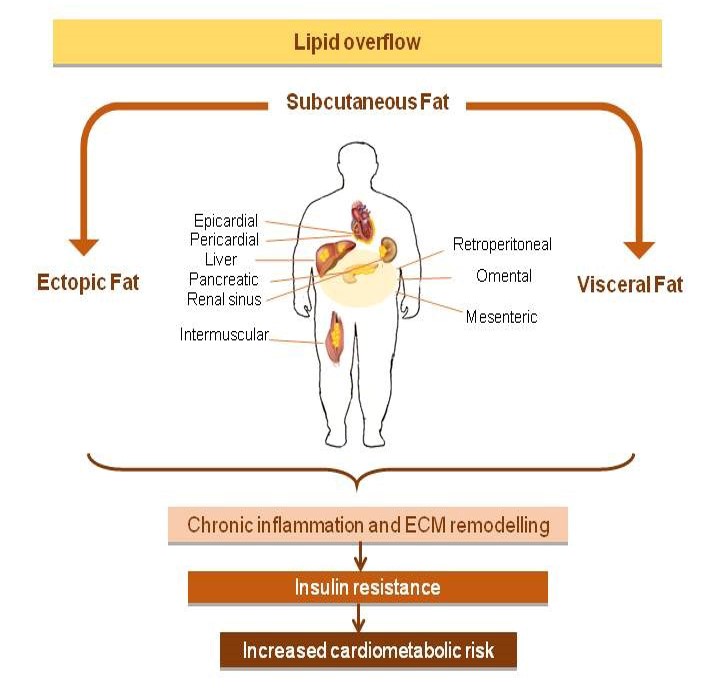 Figure 1: Excess visceral and ectopic fat accumulation is causally related to the development of insulin resistance, but is also a marker of a dysfunctional subcutaneous adipose tissue being unable to appropriately store the energy excess. Figure 1: Excess visceral and ectopic fat accumulation is causally related to the development of insulin resistance, but is also a marker of a dysfunctional subcutaneous adipose tissue being unable to appropriately store the energy excess. |
Another factor controlling the WAT expansion is the regulation of lipid flux in adipocytes. Lipoprotein Lipase (LPL), which is responsible of circulating triglycerides breakdown and Free Fatty Acids (FFAs) delivery, has been shown to be abnormally regulated in obesity independent of insulin [31], and to have a depot-specific expression and activity. Whereas the abdominal SC fat depot is characterized by a ready FFAs uptake and storage after a meal and high lipid turnover (i.e., lipolysis) acting as short-term energy store, the lower-body fat exhibits a reduced lipid turnover rate and sequesters lipids that would otherwise be directed towards non-adipose tissues, thereby acting as a protective ‘metabolic sink’ [32]. However, differences in energy storage between SC and visceral adipose tissue following meal ingestion have not been directly assessed since measurements of arteriovenous concentrations gradients across human visceral adipose tissue is not feasible.
Although the contribution of FFAs released by visceral depots appears to be insufficient in causing systemic insulin resistance [33] and the SC was shown to be the major source of circulating FFAs, omental fat is more sensitive to the lipolytic effects of catecholamines [34] and less responsive to the anti-lipolytic effects of insulin than SC depots [31].
Hypertrophic WAT is characterized by chronic inflammation due to increased inflammatory adipokines secretion and infiltrating adaptive and innate immune cells. This inflammatory profile is mostly typical of visceral rather than SC depot [35], which rather maintains its dominance in secreting adiponectin, an adipokine exerting anti-inflammatory and multiple beneficial effects on metabolism [36], although at a much lower level in obese states. With regard to SC depot differences, it has been recently demonstrated in vivo that IL-6 release from gluteo-femoral fat is markedly lower than from the abdominal fat in both genders [19]. However, human abdominal SC adipose tissue is divided by the Scarpa’s fascia into deep and superficial layers that have different structural and functional properties. It has been demonstrated that deep abdominal SC fat exhibits a more inflammatory profile and an increased proportion of small adipocytes [37].
In obese WAT, the immune cells infiltrates are mainly represented by macrophages [38], which also exhibit a phenotypic switch toward the classically activated, proinflammatory phenotype (M1), with a parallel reduction in alternatively activated, anti-inflammatory macrophage phenotype (M2) [39]. Even this process occurs in different extents and through different mechanisms in various fat depots [23].
Despite the belief that inflammatory signals play a fundamentally deleterious role in obesity complications, there is evidence in rodents that WAT inflammation should be also regarded as an adaptive response required for proper adipose tissue remodeling and expansion and contributing to a visceral fat barrier against gut-derived endotoxin, again with interdepot differences [40].
WAT expansion require extracellular matrix remodelling orchestrated by growth angiogenic factors and proteolytic enzymes. WAT fibrosis, which occurs as part of an adaptive process and in response to inflammation and local hypoxia, has been increasingly appreciated as a novel player in WAT dysfunction [39]. Indeed, over the course of obesity development, WAT fibrosis may limit further WAT expansion and lipid storage flexibility favoring ectopic over local storage of lipids [41]. Indeed, peri cellular, rather than total fibrosis, especially in the visceral compartment, was found related to both total and regional adiposity as well as to cardiometabolic alterations [42]. We showed also that omental WAT fibrosis is consistent with a higher degree of insulin resistance assessed by euglycemic clamp in severe obesity and confirmed in vivo the link between collagen deposition and issue hypoxia and inflammation [39].
The expansion of WAT requires also angiogenesis which could be driven by metabolic, developmental or hypoxic cues secondary to adipocyte growth. However, adipocyte hypertrophy entails an increased intercapillary distance and a lower capillary density per adipocyte, resulting in relatively decrease in blood perfusion to each adipocyte [43]. Therefore, it has been proposed that the expansion of adipose tissue mass during obesity development may lead to a relative oxygen deficit in adipose tissue, due to uncoordinated or insufficient angiogenesis. In relation to adipose depots differences, SC fat has a higher capillary density and higher angiogenic capacity compared to visceral depots, although both decrease when obesity develops [44]. Moreover, SC but not visceral adipose tissue angiogenic capacity negatively correlates with insulin sensitivity [44]. However, it should be taken into account that enlargement of adipocyte sizes may also result in decreased metabolic demands so that adipocytes may not necessarily suffer from hypoxia [45].
Ectopic fat depots and their local effects
Ectopic fat is defined by excess adipose tissue in locations not classically associated with adipose tissue storage [46].
Whereas intraperitoneal fat (including omental, mesenteric and epiploic depots) is considered an ectopic fat depot with predominantly systemic effects, epicardial, perivascular, renal sinus and intermuscular fat are ectopic fat depots with primarily local effects. Although the accretion of these smaller visceral depots have apparently scarce systemic impact, their pathophysiological significance is rapidly emerging supported by multiple lines of evidence from basic and translational science as well as from epidemiology.
Epicardial fat, which is in direct contact with the surface of the myocardium and coronary vessels with no separation by a physical fascia, as well as perivascular adipose tissue, which is contiguous to adventitia layer, have been described as having a dichotomous role, both protective and negative according to diverse clinical settings [47]. Indeed, epicardial adipose tissue has been hypothesized to act as mechanical cushion protecting the underlying cardiac structures, as an immediate source of FFAs in case of high cardiac demand conditions and as depot capable of buffering FFAs excess that could negatively affect the electric impulse propagation. Similarly, previous translational work has shown that perivascular adipose tissue possesses anticontractile properties and secretes substances with beneficial vasoactive properties, including adiponectin and the adipocyte-derived relaxing factor. However, these positive properties are abolished with the development of obesity. These derangements appear to be related to infiltration of the adipose tissue by macrophages and upregulation of bioactive molecules that could exert their effects through paracrine and vasocrine mechanisms [48,49]. Consistent with this finding, epicardial fat of subjects affected by coronary artery disease was found to be more abundant and to exhibit greater oxidative stress and inflammatory burden than SC fat [50-52]. In addition, in obese patients EAT amount was associated with increased left ventricular mass, right ventricular cavity size, atrial enlargement and diastolic dysfunction [53-55]. It has also been reported an association between EAT volume and atrial fibrillation independent of common atrial fibrillation risk factors and obesity [56]. In this regard, EAT might promote fibrosis in the neighbouring myocardium through the secretion of profibrotic factors including inflammatory cytokines, growth factors and matrix metalloproteinases [49,57]. When atrial myocardium was incubated with the secretome of EAT, but not of parasternal SC fat, obtained from CAD patients in an ex vivo model, it developed massive fibrosis through the transformation of fibroblasts into myofibroblasts [49].
Similarly, perivascular fat, under pathological conditions, accumulates and becomes dysfunctional, showing a marked proinflammatory, proliferative and angiogenic profile [58].
Other local fat depots that have gained increasing attention in the last years are the intermuscular fat, whose accretion is not only linked to impairment of muscle mobility and function [20,59], but also possibly to muscular insulin resistance development [60], and renal sinus adipose tissue, which has been independently associated with hypertension, chronic kidney disease [61] and microalbuminuria [62]. Recent findings also suggest the involvement of periprostatic adipose tissue in prostate cancer progression [63], and of bone marrow adipose tissue in hematopoiesis regulation and in the pathophysiology of myeloma and bone metastases [64].
Ectopic lipids storage and lipotoxicity
When obesity develops and the circulating lipids exceed storage capacity of adipose tissue, an abnormal intracellular retention of lipids within non-adipose tissues occurs. This process may involve liver, skeletal and cardiac muscles and pancreas [65]. Excess lipid storage during pathological conditions, such as obesity, may lead to an imbalance in lipid homoeostasis that lead to cell dysfunction or death, a phenomenon called lipotoxicity [66]. Bioactive lipid metabolites (e.g., ceramides, diacylglycerol and long-chain fatty acyl-CoAs) accumulation is now known to be a mechanism that contributes to organ injury in the context of metabolic diseases [66].
In human studies, intrahepatic and intramyocellular lipid content emerged as much stronger predictors of insulin resistance than circulating fatty acids, suggesting that intracellular lipids may impair insulin signaling and cause insulin resistance [67]. Similarly, accumulation of fat in the heart has been associated with cardiac dysfunction and heart failure, possibly contributing to diabetic cardiomyopathy development [68]. Finally, evidence that pancreatic steatosis has a pathogenetic role in type 2 diabetes by impairing β-cell function is also emerging [47].
Concluding Remarks
BMI and total body fat mass are important predictors of metabolic derangements at the population level. However, body fat distribution and accumulation in ectopic sites have been recognized as key factors in adipose tissue dysfunction, and thus crucial determinants of obesity-related insulin resistance and cardiometabolic complications at the individual level.
This apparent compartmentalization of WAT and ectopic partitioning of excess dietary lipids which are influenced by genetic predisposition, environment, gender and age, play a critical role in determining the obesity phenotype and, thus, the susceptibility to obesity complications. Inter- and intradepot heterogeneity of adipose tissue which takes origin from the developmental diversity adipocyte precursors in different depots, is then amplified during chronic energy balance challenge by cell-autonomous differences in adipogenetic properties, lipid metabolism and adipokine secretion profile of adipocytes. Understanding the pathogenesis of ectopic and lipid mediated organ damage will provide new insight into the pathogenesis of obesity complications and consequently will help identify new targets for therapeutic intervention.
References
- Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772-783.
- Chudek J, Wiecek (2006) Adipose tissue, inflammation and endothelial dysfunction. Pharmacol Rep 58: 81-88.
- Guglielmi V, D’Adamo M, Bellia A, Ciotto RT, Federici M, et al. (2015) Iron status in obesity: An independent association with metabolic parameters and effect of weight loss. Nutr Metab Cardiovasc Dis 25: 541-547 .
- Prentice AM, Jebb SA (2001) Beyond body mass index. Obes Rev 2: 141-147.
- Goossens GH (2017) The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts 10: 207-215.
- Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, et al. (2004) Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord 28: 402-409.
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, et al. (2005) Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 366: 1640-1649.
- Coutinho T, Goel K, Corrêa de Sá D, Kragelund C, Kanaya AM, et al. (2011) Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 57: 1877-1886.
- Lombardi F, Gullotta F, Columbaro M, Filareto A, D’Adamo M, et al. (2007) Compound heterozygosity for mutations in LMNA in a patient with a myopathic and lipodystrophic mandibuloacral dysplasia type A phenotype. J Clin Endocrinol Metab 92: 4467-4471.
- Guglielmi V, D’Adamo M, D’Apice MR, Bellia A, Lauro D, et al. (2010) Elbow deformities in a patient with mandibuloacral dysplasia type A. Am J Med Genet A 152: 2711-2713.
- McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, et al. (2010) Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring) 18: 926-931.
- Pramyothin P, Karastergiou K (2016) What Can We Learn from Interventions That Change Fat Distribution? Curr Obes Rep 5: 271-281.
- Hernandez TL, Bessesen DH, Cox-York KA, Erickson CB, Law CK, et al. (2015) Femoral lipectomy increases postprandial lipemia in women. Am J Physiol Endocrinol Metab 309: 63-71.
- Cameron AJ, Magliano DJ, Söderberg S (2013) A systematic review of the impact of including both waist and hip circumference in risk models for cardiovascular diseases, diabetes and mortality. Obes Rev 14: 86-94.
- Wells JC (2007) Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 21: 415-430.
- Horber FF, Zürcher RM, Herren H, Crivelli MA, Robotti G, et al. (1986) Altered body fat distribution in patients with glucocorticoid treatment and in patients on long-term dialysis. Am J Clin Nutr 43: 758-769.
- Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, et al. (2005) Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res 13: 2139-2145.
- Hilton C, Karpe F, Pinnick KE (2015) Role of developmental transcription factors in white, brown and beige adipose tissues. Biochim Biophys Acta 1851: 686-696.
- Pinnick KE, Nicholson G, Manolopoulos KN, McQuaid SE, Valet P, et al. (2014) Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes 63: 3785-3797.
- Guglielmi V, Maresca L, D’Adamo M, Di Roma M, Lanzillo C, et al. (2014) Age-related different relationships between ectopic adipose tissues and measures of central obesity in sedentary subjects. PLoS One 9: 103381.
- Lee MJ, Wu Y, Fried SK (2013) Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34: 1-11.
- Anand SS, Tarnopolsky MA, Rashid S, Schulze KM, Desai D, et al. (2011) Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6: 22112.
- Kwok KH, La KS, Xu A (2016) Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med 48: 215.
- Goossens GH (2008) The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94: 206-218.
- Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, et al. (2005) Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab 288: 267-277.
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, et al. (2006) Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA 103: 6676-6681.
- Guglielmi V, D’Adamo M, Menghini R, Cardellini M, Gentileschi P, et al. (2017) MicroRNA 21 is up-regulated in adipose tissue of obese diabetic subjects. Nutr Healthy Aging 4: 141-145.
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM (2009) Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27: 2563-2570.
- Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, et al. (2014) Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab 20: 1049-1058.
- Frayn KN (2001) Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc 60: 375-380.
- Wajchenberg BL (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697-738.
- Karpe F, Pinnick KE (2015) Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol 11: 90-100.
- Jensen MD (2006) Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity (Silver Spring) 1: 20-24.
- Arner P (1999) Catecholamine-induced lipolysis in obesity. Int J Obes Relat Metab Disord 23: 10-13.
- Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, et al. (2011) Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res 52: 480-488.
- Alvehus M, Burén J, Sjöström M, Goedecke J, Olsson T (2010) The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 18: 879-883.
- Cancello R, Zulian A, Gentilini D, Maestrini S, Della Barba A, et al. (2013) Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity (Silver Spring) 21: 2562-2570.
- Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98-107.
- Guglielmi V, Cardellini M, Cinti F, Corgosinho F, Cardolini I, et al. (2015) Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes 5: 175.
- Asterholm IW, Tao C, Morley TS, Wang QA, Delgado-Lopez F, et al. (2014) Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 20: 103-118.
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, et al. (2009) Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575-1591.
- Michaud A, Tordjman J, Pelletier M, Liu Y, Laforest S, et al. (2016) Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int J Obes (Lond) 40: 1823-1831.
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, et al. (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58: 718-725.
- Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, et al. (2011) Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 123: 186-194.
- Goossens GH, Blaak EE (2015) Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne) 6: 55.
- Britton KA, Fox CS (2011) Ectopic fat depots and cardiovascular disease. Circulation 124: 837-841.
- Guglielmi V, Sbraccia P (2017) Epicardial adipose tissue: at the heart of the obesity complications. Acta Diabetol.
- Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, et al. (2009) Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661-1670.
- Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, et al. (2015) Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of apo-fibrokines. Eur Heart J 36: 795-805.
- Salgado-Somoza A, Teijeira-Fernández E, Rubio J, Couso E, González-Juanatey JR, et al . (2012) Coronary artery disease is associated with higher epicardial Retinol-Binding Protein 4 (RBP4) and Lower Glucose Transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin Endocrinol (Oxf) 76: 51-58.
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, et al. (2003) Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108: 2460-2466.
- Iwayama T, Nitobe J, Watanabe T, Ishino M, Tamura H, et al. (2014) Role of epicardial adipose tissue in coronary artery disease in non-obese patients. J Cardiol 63: 344-349.
- Iacobellis G, Leonetti F, Singh N, Sharma AM (2007) Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol 115: 272-273.
- Iacobellis G, Cotesta D, Petramala L, De Santis V, Vitale D, et al. (2010) Intracoronary adiponectin levels rapidly and significantly increase after coronary revascularization. Int J Cardiol 144: 160-163.
- Guglielmi V, Maresca L, Lanzillo C, Marinoni GM, D’Adamo M, et al. (2016) Relationship between Regional Fat Distribution and Hypertrophic Cardiomyopathy Phenotype. PLoS One 11: 0158892.
- Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, et al. (2011) Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J 75: 2559-2565.
- Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, et al. (2012) Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 126: 2324-2334.
- Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, et al. (2012) The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia 55: 1514-1525.
- Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC (2010) Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging 14: 362-366.
- Addison O, Marcus RL, Lastayo PC, Ryan AS (2014) Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014: 309570.
- Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, et al. (2011) Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension 58: 784-790.
- Wagner R, Machann J, Lehmann R, Rittig K, Schick F, et al. (2012) Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia 55: 2054-2058.
- Ribeiro RJ, Monteiro CP, Cunha VF, Azevedo AS, Oliveira MJ, et al. (2012) Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol Biochem 29: 233-240.
- Hardouin P, Rharass T, Lucas S (2016) Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue? Front Endocrinol (Lausanne) 7: 85.
- Szendroedi J, Roden M (2009) Ectopic lipids and organ function. Curr Opin Lipidol 20: 50-56.
- Schaffer JE (2003) Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14: 281-287.
- Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852-871.
- Loher H, Kreis R, Boesch C, Christ E (2016) The Flexibility of Ectopic Lipids. Int J Mol Sci 17: 1554.
 LOGIN
LOGIN REGISTER
REGISTER.png)
