Case Report
Performance of iPhone Hyperemesis Gravidarum Care App
Edwin Korouri1, Kimber MacGibbon2, Michelle Chan3, Leonides Guba3, Lorence Dela Cruz3, William Leung3, John Wang3, Kirsten Jensen1 and Marlena S Fejzo1,4,*
1University of California, Los Angeles, California, USA
2HER (Hyperemesis Education & Research) Foundation, Damascus, Oregon, USA
3UCLA Health Information Technology, Los Angeles, California, USA
4University of Southern California, Los Angeles, California, USA
*Corresponding author: Marlena S Fejzo, University of California, Los Angeles, California, USA, Tel: (310) 206-1408; E-mail: mfejzo@mednet.ucla.edu
Citation: Korouri E, MacGibbon K, Chan C, Guba Leonides, Cruz LD (2019) Performance of iPhone Hyperemesis Gravidarum Care App. J Clinical Case Rep Case Stud 2019: 21-27. doi:
Received: 11 January, 2019; Accepted: 28 February, 2019; Published: 08 March, 2019
Précis
Usage of iPhone application improves accuracy in defining symptom level, communication between patients and providers, and treatment of Hyperemesis Gravidarum for both patient and providers.
Abstract
Introduction: Hyperemesis Gravidarum (HG), severe nausea and vomiting in pregnancy, occurs in up to 2% of pregnancies, leads to significant weight loss, prolonged malnutrition and dehydration, and is associated with both maternal and fetal morbidity. Patients with HG often need help monitoring their care due to being ill throughout pregnancy. We created a mobile application to help HG patients track their symptoms and medications with the objective of improving care and communication.
Methods: The study was performed at the University of California, Los Angeles (UCLA). Patients were invited to participate in the study at their doctor visit at UCLA and/or through social media. Interested participants were asked to use an iPhone app to record symptoms, intake, and medications for 7 days prior to their next prenatal appointment. Copies of data were given to their provider, and both the patient and provider completed an app success survey at the appointment. Feedback was requested from both the patient and provider on the App’s effectiveness for defining symptom level, improving communication, and improving treatment of HG, as well as any suggestions for improvements for the HG Care App.
Results: Thirty-six patients participated in symptom tracking and provided feedback, and 6 providers gave feedback on the App. Data analysis showed that patients felt positive about the App’s ability to accurately define symptom levels (92%), improve communication (66%), and improve care (61%). Providers unanimously thought the HG Care App was accurate in defining symptom levels and was useful in improving communication, and most (67%) found it useful in improving care. Common feedback regarding the App included: being tedious to fill out (22%), being able to more easily explain symptoms to one’s provider (14%), and adding a daily reminder to input symptoms in future versions (11%).
Discussion: A majority of both patients and providers are proponents of using the HG mobile application for accurately defining symptom levels and improving quality of care and communication. Users requested improvements to App UI, and additional features be included in future versions. Results indicate that mobile apps have potential to be effective tools towards treating HG and severe NVP.
Keywords: Mobile health; Hyperemesis Gravidarum; iPhone application
Quick Points
- An iPhone app, “HG Care App,” was developed to improve accuracy in defining symptom levels of Hyperemesis Gravidarum (HG), communication between providers and patients with HG, and treatment of HG.
- Patients were asked to use the HG Care App to record data regarding symptoms, medication, and other factors in anticipation of an upcoming prenatal visit, after which patients and providers rated their experience using the App.
- Feedback from both patients and providers who filled out survey shows that App helped with accuracy of diagnosis, treatment, and improved communication between patients and providers.
- The HG Care App can have positive effects in the overall treatment of HG
Introduction
Nausea and vomiting of pregnancy (NVP) affects between 50-90% of all pregnant women [1]. Hyperemesis Gravidarum (HG), the most severe form of NVP, occurs in 0.3-2% of pregnancies and leads to significant weight loss, malnutrition, dehydration, electrolyte imbalance, and ketonuria [2,3]. HG is commonly associated with maternal morbidity such as Wernicke’s encephalopathy, [4] renal and liver function abnormalities, [5,6] esophageal rupture, [7] and post-partum post-traumatic stress [8]. HG is also associated with a 4-fold increased risk of adverse fetal outcome including low birth weight, intrauterine growth restriction, preterm delivery, fetal and neonatal death, and a 3-fold increased risk of neurodevelopmental delay in children [9,10]. Therapeutic terminations occur in as many as 15% of women and maternal deaths still occur in extreme cases [11,12]. Thus, there is a critical need to better manage patients in order to reduce adverse maternal and fetal outcomes.
It is currently estimated that as many as 18% of women in the US take medication for NVP [13]. In a prior study of 254 women with HG, subjects were treated with intravenous fluids for HG; the most common antiemetic treatments were ondansetron (79%), promethazine (73%), and metoclopramide (55%). The self-reported effectiveness in relieving symptoms was approximately 50% for ondansetron, with less than 20% reporting promethazine was effective, and less than 10% reporting metoclopramide was effective [14]. As these results are based on self- reports, it is necessary to collect clinical data on patients with HG to confirm symptom improvement following treatment. A recent Cochrane review finds there is insufficient data on the efficacy of any medications used to treat HG, confirming the need for improving data collection in this area to be able to improve treatment efficacy and quality of care for patients with HG [15]. An accurate tool that uniformly collects data on a patient’s symptoms and treatment effectiveness in real time is needed to measure medication effectiveness such that patients and providers can observe if symptoms are improving.
To address this unmet need, we have developed the HG Care App to collect clinical data on symptoms, treatments, and outcomes of patients with HG daily. Furthermore, the App was developed to allow providers to review and adjust treatment according to the individual patient’s needs, improve communication between patients and their caretakers, and alert patients to concerning changes such as weight loss or dehydration that may necessitate immediate care. This study was performed to determine the success of the App in defining symptom levels, improving communication with healthcare providers, and improving overall quality of care as well as identifying areas for improvement.
Methods
HG Care App [16]
For HG patients, the App functions to track medication, food, fluid, and vitamin intake; symptoms including nausea, vomiting, retching, urination, bowel movements, and weight; and changes in NVP levels using the PUQE Score [17] in addition to other factors related to patient health and wellbeing in real time and calculates a HyperEmesis Level Prediction (HELP) score. The HELP score contains all the elements of the validated PUQE Score but also includes items such as medication and food intake and tolerance, urination frequency, psychosocial functioning, and weight, in order to better assess antiemetic effectiveness in improving symptoms of dehydration, starvation, debility, and weight loss, which are characteristic of HG. Additionally, Google Analytics was used to track the number of times patients used the “Share Report” function within the insight page to share or print the report for their care provider.
Participants
Between September-December, 2017, University of California, Los Angeles (UCLA) patients with severe NVP and HG were invited to test the HG Care App at a prenatal care appointment by their obstetrician, or were recruited by social media via posts on the UCLA Health website at https://connect.uclahealth.org/2017/10/10/researchers-testing-new-app-to-help-track-extreme-morning-sickness-during-pregnancy/ and the Hyperemesis Education and Research (HER) Foundation facebook page at https://www.facebook.com/HERFoundation/. The HER Foundation is the largest foundation for support, education, and research regarding hyperemesis gravidarum in the United States. Those who were interested in using the HG Care App contacted the study coordinator and were screened to determine whether they had an iPhone and symptoms of severe NVP or HG. Pregnant women who met these criteria were given instructions to download the iPhone App.
Procedure
After being prompted to download the HG Care App on their iPhone, patients were asked to: 1) use the App daily for 7 days prior to next provider visit 2) print 2 copies of the report (insight page) within 24 hours of provider visit 3) fill out/sign App success survey at appointment, and ask their provider to do so as well, and 4) return signed survey(s) via scan/email or online survey to researchers.
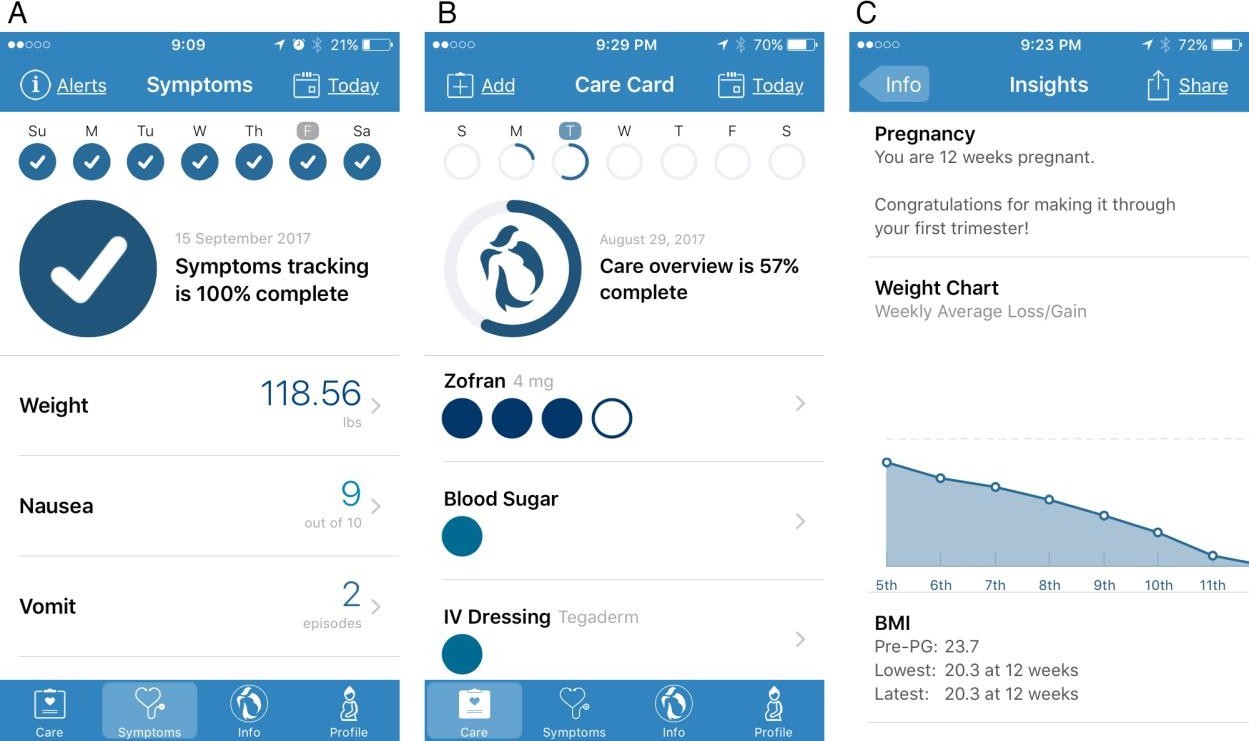
Daily App use included completing the “Symptoms” section of the App (Figure 1A). Patients were also instructed to complete prescribed and self-care behaviors within the “Care” section (Figure 1B). Patients were given a completion status at the top of each data submission page to encourage and remind completion of timely reports. All recorded elements in the App were chronologically separated by days of the week. “Insight” pages that users brought to their prenatal visit included a summary of all self-reported data for physicians and patients to review together, including graphical summaries charting symptoms versus medication usage, food and fluid intake, and weight (Figure 1C). The Insight pages also provided suggestions given to the patient in the form of alerts based on data collected such as talking to your provider about getting thiamin if unable to keep food and vitamins down. After a patient utilized the “Share Report” function within the insight page, Google Analytics recorded the event as well as the number of unique users that utilized this function.
|
Figure 1: HG Care App screenshots. A) Representative display of completed symptoms page. Users were instructed to self-report symptoms experienced daily for 7 days leading up to their appointment with an obstetrician. B) Screenshot representative of incomplete care page, where users recorded factors related to medication/treatment, self-care behavior, and diet. C) Representative view of insight page featuring graphical representation of weight changes and summary of BMI history based on user-submitted data. Data inputted in symptoms and care pages were summarized on insight page and later shared with obstetrician at the time of their appointment. |
Feedback from both patient and provider on the App’s effectiveness for defining symptom level, improving communication, and improving treatment of HG was recorded at the prenatal visit. Patients and providers were asked to answer the following questions “1) Did you find the App accurate in defining symptom level? 2) Did you find the App useful in improving communication? And 3) Did you find the App useful in improving care?” with the possible answers as “Yes, maybe, no, or not sure.” Both parties were also given an optional free response section to suggest necessary App improvements. Responses to these four questions were collected and analyzed to determine the App’s success in improving treatment of HG, and to identify areas for improvement.
Users’ app download and usage statistics were collected from September-December, 2017, using data provided by iTunes Connect, the Apple App Store’s platform for recording a user’s app download and usage statistics.
This study was approved by the Institutional Review Board UCLA IRB#17-000147.
Results
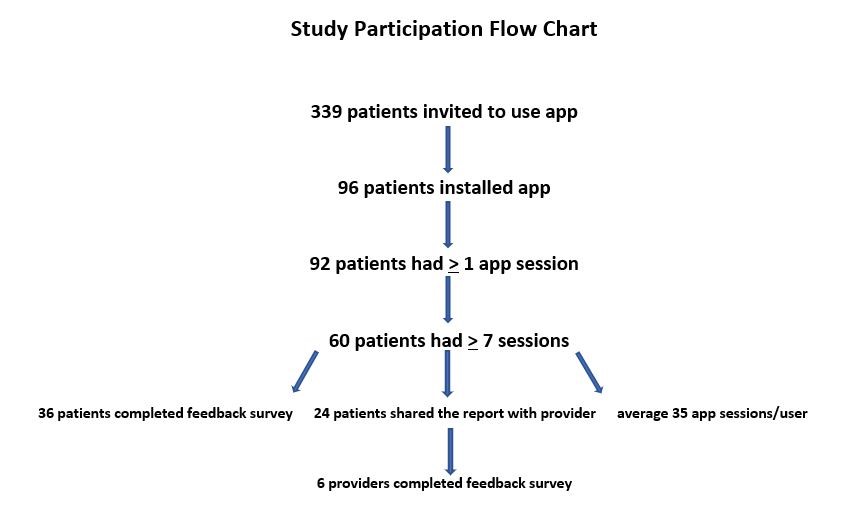
During the study period, 339 patients were invited to use the App, and 96 (28.32%) installed the HG Care App (Figure 2). Of the 96 patients who installed the App, 92 (95.83%) had at least one app session—defined as opening and closing the App, and 60 (62.50%) had 7 or more sessions, thereby completing the task to input data for 1 week leading up to their next prenatal appointment. Thirty-six of the 60 patients who inputted data for 7 or more sessions (60%) completed the feedback survey. Among the 60 patients who had a minimum of 7 sessions, the average user had 34.5 sessions, with a range between 7 and 178 sessions. According to data provided by Google Analytics for Firebase for recording app usage and user engagement statistics, 24 unique users utilized the “Share Report” feature 82 times cumulatively from September-December, 2017, for an average of 3.4 shares per user. Demographic data of participants was not collected.
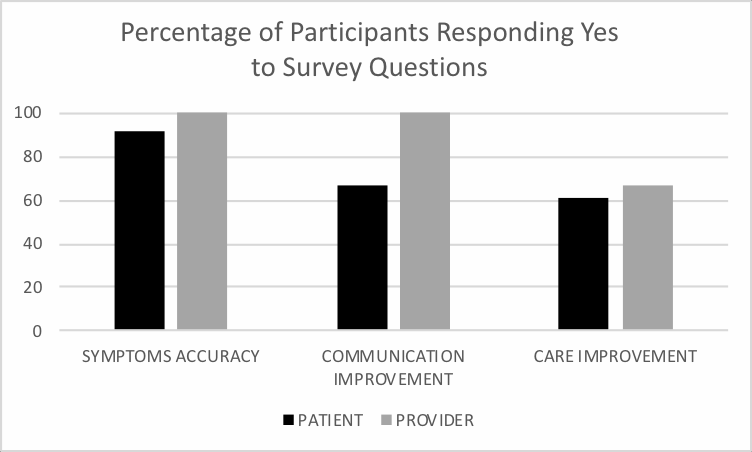
Thirty-three patients (91.67%) responded “Yes” to finding the App accurate in defining symptom levels. 1 patient responded “Maybe,” 1 responded “No,” and 1 responded “Unsure” (Figure 3). Six provider responses were collected. All 6 of the providers who filled out the survey answered “Yes” to finding the App accurate in defining symptom levels (Figure 3).
|
Figure 2: Flow of study participation. |
|
Figure 3: Graph showing percentage of “Yes” responses to survey questions following provider visit. Percentage of 36 patients (and 6 providers) who completed 7 days of data input and reviewed insight page with provider that responded “Yes” to survey questions. Questions were as follows: “1. Did you find the App accurate in defining symptom level? 2. Did you find the App useful in improving communication? 3. Did you find the App useful in improving care?”. |
When asked if they found the App was useful in improving communication, 24 patients (66.67%) responded “Yes,” 3 patients responded “Maybe,” 3 responded “No,” and 6 responded as “Unsure.” All 6 providers found the App was useful in improving communication.
Finally, 22 patients (61.11%) responded “Yes” to finding the App useful in improving care. 6 patients responded “Maybe,” 6 responded “No,” and 2 responded as “Unsure.” Four providers found the App useful in improving care, 1 responded “Maybe,” and 1 responded “Unsure.”
Following standard questions, patients and providers were prompted to give open-ended feedback to improve future versions of the HG Care App. The most frequent comment, made by 8 patients, was that the App was tedious and/or difficult to fill out, especially on days when they were most ill. Other frequent comments in the feedback portion of the survey involved appreciating being able to view areas of improvement (6 patients), addressing where further changes need to be made (6 patients), and having an easier time explaining symptoms to their provider (3 patients). Common suggestions for future updates included: adding a feature to add one-time medication (5 patients), adding ability to track hospitalization and/or ER care (3 patients), and having the App track and alert based on trends rather than day-to-day comparisons (3 patients).
Discussion
This is the first study of an application developed specifically for HG care, to the best of our knowledge. The data shows that the majority of patients and providers are proponents of using the HG Care App for accurately defining symptom levels and improving quality of care and communication. Tracking symptoms, medication taken, and other markers daily through the HG Care App may serve as a valuable tool in the treatment of HG.
Patient feedback does indicate that there is room for improvement for the App.
Addressing features and requests frequently submitted by users within the open-ended feedback portion of the survey may further increase positive responses to surveys from patients and encourage patients to continue to input data daily. Future versions also aim to integrate with popular electronic health record systems so data can be reviewed by healthcare providers more efficiently as well.
However, it is difficult to predict whether these patient-suggested improvements would increase positive responses—or responses in general—from providers. Approximately one-third of HG patients describe their providers as “uncaring” or “did not understand how sick they were” [11]. This attitude is a common problem for patients with HG, and was an impetus for creating the App—having an uncaring provider is a major indicator for therapeutic termination of planned pregnancies due to HG [11]. In addition, providers are often busy with other patients, or do not have a thorough understanding of HG or enthusiasm about mobile healthcare. These are often limitations that are out of the control of researchers, but there is hope that incoming healthcare providers will convey more enthusiasm and/or understanding of technology and mobile healthcare’s effectiveness in both managing and treating HG, and in healthcare in general [18]. Still, most participants found the App accurate in defining symptom levels, so if used universally by researchers, it may be able to resolve many of the issues in the aforementioned Cochrane review such as: enhanced analyses on medication effectiveness, the need for more studies on HG, improved data collection, and a tool to provide uniformity between different research studies.
The patient-submitted data on symptoms, weight, and other personal qualities were automatically integrated into the HELP Score within the App. Within the insight tab, HELP Scores were tracked against treatment for an overview of treatment efficacy, which could be used to determine if changes in treatment should be considered. Such data-collection can be used in future studies of medication effectiveness and in the development of treatment algorithms for improvement of both treatment and outcomes.
However, patient demographic data was not collected as part of the study. In addition, the study was restricted to iPhone users who were voluntary participants recruited through UCLA and/or social media postings, primarily a HER Foundation Facebook group. These stand as limitations to the study, as we are unable to conclude if the results of the experiment can be generalized to a larger patient population of women with HG or severe NVP.
Finally, it is important to clarify apparent incongruence in the data provided by Google Analytics and the final number of completed surveys. According to Google Analytics, 24 participants used the “Share Report” feature, while 36 total surveys were completed. This may be that a handful of users did not use the “Share Report” feature, opting instead to show their providers the insight page as it appears on their mobile phones.
Conclusion
Few, if any, mobile apps have been developed to improve care, communication, and treatment of HG. Mobile-healthcare is a rapidly emerging sub-field within healthcare, and mobile apps have potential to be valuable tools towards treating HG and severe NVP as they are with other illnesses. The feedback received from patients using the HG Care App shows great promise, but improvements can still be made to help both patients and providers. In the future, it would be beneficial to develop an Android version of the HG Care App, as well as gather data on patient demographics to be able to generalize experimental conclusions. Additionally, the effectiveness of medications and changes in care made based on App data collection should be evaluated in future studies. Finally, a study to determine whether the HG Care App is effective in reducing rehospitalization would be beneficial in further evaluating its utility in a clinical setting.
Conflict of Interest:
The authors have no conflicts of interest to disclose.
Acknowledgements
None.
Contribution
Contributors who are not included as authors are None.
References
- Clark SM, Costantine MM, Hankins GD (2012) Review of NVP and HG and Early Pharmacotherapeutic Intervention. Obstet Gynecol Inte 2012: 8.
- Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG (2005) Hyperemesis gravidarum, a literature review. Hum Reprod update. 11: 527-539.
- Kallen B (1987) Hyperemesis during pregnancy and delivery outcome: a registry study. Eur J Obstet Gynecol Reprod Biol 26: 291-302.
- Chiossi G, Neri I, Cavazzuti M, Basso G, Facchinetti F (2006) Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 61: 255-268.
- Hill JB, Yost NP, Wendel GD, Jr (2002) Acute renal failure in association with severe hyperemesis gravidarum. Obstet Gynecol 100: 1119- 1121.
- Adams RH, Gordon J, Combes B (1968) Hyperemesis gravidarum. I. Evidence of hepatic dysfunction. Obstet Gynecol 31: 659-664.
- Liang SG, Ooka F, Santo A, Kaibara M (2002) Pneumomediastinum following esophageal rupture associated with hyperemesis gravidarum. J Obstet Gynaecol Res 28: 172-175.
- Fejzo MS, Poursharif B, Korst LM, Munch S, MacGibbon KW, et al. (2009) Symptoms and Pregnancy Outcomes Associated with Extreme Weight Loss among Women with Hyperemesis Gravidarum. J Women's Health 18: 1981-1987.
- Fejzo MS, Magtira A, Schoenberg FP, MacGibbon K, Mullin P, et al. (2013) Antihistamines and other prognostic factors for adverse outcome in hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 170: 71-76.
- Fejzo MS, Magtira A, Schoenberg FP, Macgibbon K, Mullin PM (2015) Neurodevelopmental delay in children exposed in utero to hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 189: 79-84.
- Poursharif B, Korst LM, Macgibbon KW, Fejzo MS, Romero R, et al. (2007) Elective pregnancy termination in a large cohort of women with hyperemesis gravidarum. Contraception 76: 451-455.
- Fejzo MS, MacGibbon K, Mullin PM (2016) Why are Women Still Dying from Nausea and Vomiting of Pregnancy? Gynecol Obstet Case Rep 2: 25-28.
- Piwko C, Koren G, Babashov V, Vicente C, Einarson TR (2013) Economic burden of nausea and vomiting of pregnancy in the USA. J Popul Ther Clin Pharmacol 20: e149-160.
- Lerner L, Hayes TG, Tao N, Krieger B, Feng B, et al. (2015) Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 6: 317-324.
- Boelig RC, Barton SJ, Saccone G, Kelly AJ, Edwards SJ, et al. (2017) Interventions for treating hyperemesis gravidarum: a Cochrane systematic review and meta- analysis. J Matern Fetal Neonatal Med: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 1-14.
- https://itunes.apple.com/app/id1148105670?fbclid=IwAR23-ChS5uHJboRLAhjXHRDQCMspx4H4RtRfPewxLekpS3pCi14XiJi6quQ
- Koren G, Piwko C, Ahn E, R Boskovic, C Maltepe, et al. (2005) Validation studies of the Pregnancy Unique- Quantification of Emesis (PUQE) scores. J obstet gynaecol 25: 241-244.
- Payne KFB, Wharrad H, Watts K (2012) Smartphone and medical related App use among medical students and junior doctors in the United Kingdom (UK): a regional survey. BMC Med Inform Decis Mak 12: 121.
 LOGIN
LOGIN REGISTER
REGISTER.png)