Research Article
Recovery of Cyprinus carpio (Ornamental Koi Carp) Experimentally Infected with Aeromonas hydrophila through Phytotherapy
Rajagopal Bhuvaneswari1*, Narasimman Manickam2,3 and Perumal Santhanam2
1Fish Disease Diagnostic Laboratory, Department of Zoology, Ayyanadar Janaki Ammal College, Sivakasi, Tamil Nadu, India.
2Marine Planktonology & Aquaculture Laboratory, Department of Marine Science, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
3Crustacean Biology Laboratory, Department of Zoology, Bharathiar University, Coimbatore, Tamil Nadu, India.
*Corresponding author: Dr. Rajagopal Bhuvaneswari, Fish Disease Diagnostic Laboratory, Department of Zoology, Ayyanadar Janaki Ammal College, Sivakasi, Tamil Nadu, India, E-mail: bhuvanar3@gmail.com or drbhuvana3@gmail.com
Citation: Bhuvaneswari R, Manickam N, Santhanam P (2018) Recovery of Cyprinus Carpio (Ornamental Koi Carp) Experimentally Infected with Aeromonas Hydrophila through Phytotherapy. J Aquat Res Mar Sci 2018: 55-68. doi:https://doi.org/10.29199/ARMS.102014
Received Date: 12 October, 2017; Accepted Date: 9 October, 2018; Published Date: 16 November, 2018
Abstract
The aim of the present study was to obtain a basic knowledge of the hematology of Cyprinus carpio-Ornamental Koi Carp. The morphological features of blood cells were described according to the observations made by light and electron microscopy. Erythrocytes, thrombocytes and four types of leucocytes: lymphocytes, monocytes, heterophils and eosinophils, were distinguished and characterized. Thrombocytes are the most abundant blood cells after erythrocytes and are recognized easily from lymphocytes by morphological features and size. Heterophils and eosinophils are PAS positive. Hematological indices (RBC, WBC, PCV, Hb, MCV, MCH, MCHC and leucocyte differential count) were measured in one blood sample from experimental animal. This research was to reveal the benefit of phytotherapy instead of chemotherapeutic agents of bacterial pathogen Aeromonas hydrophila. Which in intramuscularly infected Koi carp were manifested as a hemorrhagic spot within 19.00±2.00 has a lesion which progressively increased in size as open wound measuring 1.73±0.50 cm in diameter on the 3rd day.The infected fishes were involved in the short bath treatment, healing commenced with the shrinking of the lesion and appearance of epidermis, on the third day of treatment in 15±2 days with Acorus calamus and 17±3 days with Indigofera aspalathoides. The scale formation was complete in the next 5±2 days with A. calamus and 20±3.5 days with I. aspalathoides confirming the superiority of the short bath treatment. However, with A. calamus treatment on the 15th day the values varied significantly indicating speedy recovery; the results prove the efficacy of short bath treatment with herbal residue and the increased potency of A. calamus over I. aspalathoides in combating the A. hydrophila infection in Koi carp.
Key words
Aeromonas hydrophila; Cyprinus carpio; Hematological; Infection; Phytotherapy
Introduction
Globally aquaculture is the fastest growing activity with an annual increase of about 15% per year [1]. With about 194 genera and 2070 species, cyprinids like the widest continuous distribution of any freshwater fish family throughout the world [2]. According to the FAO 1996 report, carp production is predicted to increase from 6.7 million tons in 1992 to 10.2 million tons by year 2000. Among the various fishes grown worldwide, the freshwater cyprinids that include food fishes like major carps and a number of prized ornamental fishes like koi carp comprise the largest cultivated group. Aquaculture as an industry is in a phase of rapid expansion and the intensive fish farming often leads to the emergence of infectious and parasitic diseases. Disease has become a critical factor hampering the development of carp culture in many countries. Among the various pathogens, the bacteria represent the greatest potential threat to aquaculture [3]. Carp are susceptible to wide variety pathogens including bacterial diseases like hemorrhagic disease, erythrodermatitis, enteric red mouth disease [4,5].
Most bacterial pathogens of Koi are gram negative rods and include such genera as Aeromonas and Pseudomonas [6]. Aeromonas hydrophila has also been identified as a lethal problem in Koi ponds. The bacterial infections can be severe and lethal bacteremias and septicemias are not uncommon [7]. Of these diseases motile aeromonad septicemia (MAS) caused by Aeromonas hydrophila is a devastating disease has wiped out carp farms [8] and infects fishes like cat fishes, bass and a variety of tropical ornamental fishes [9]. Antimicrobial agents like Terramycin, potassium permanganate and Malachite green are the choice drugs although complete control of a disease is yet to be achieved [10]. Synthetic chemicals and antibiotics have been used to treat bacterial infection in fishes for the last 30 years or so at least with partial success [11]. Besides most of the medicines used in aquaculture have residual effects on the environment, while some of them accumulate and get concentrated in aquatic organisms [12,13]. Few vaccines are presently available; though benign, in several cases their efficacy is questioned, at least under practical field conditions [14].
Hematological indices are important parameters for the evaluation of fish physiological status. Their changes depend on the fish species, age, the cycle of sexual maturity and health condition [15-23]. Hematological parameters are closely related to the response of the animal to the environment, an indication that the environment where fishes live could exert some influence on the hematological characteristics [24]. These indices have been employed in effectively monitoring the responses of fishes to the stressors and thus their health status under such adverse conditions. They can provide substantial diagnostic information once reference values are established under standardized inditions. Evaluation of the hemogram involves the determination of the total erythrocyte count (RBC), total white blood cell count (WBC), hematocrit (PCV), hemoglobin concentration (Hb), erythrocyte indices (MCV, MCH, MCHC), white blood cell differential count and the evaluation of stained peripheral blood films [25]. Thrombocytes have been described as the most abundant blood cells after erythrocytes. The count of red blood cells is quite a stable index and the fish body tries to maintain this count within the limits of certain physiological standards using various physiological mechanisms of compensation. Studies have shown that when the water quality is affected by toxicants, any physiological changes will be reflected in the values of one or more of the haematological parameters [26]. Blood cell responses are important indicators of changes in the internal and/ or external environment of animals.
In view of the emergence of resistant strains of pathogens such as A. hydrophila aquaculture needs new lines of antimicrobial agents since therapy will remain as one of the main means of controlling transmissible diseases in future [27]. Today, pharmaceutical companies produce more than 160 different antibiotics to combat bacterial infection [14]. However, these provide only temporary solutions to the problem of resistance because existing resistance mechanisms often rapidly adapt to accommodate the new derivates [28-30] and in the fish disease management scenario any alternative tool will be a welcome addition. In India herbal remedies play a fundamental role as the therapeutic agents in treatment of infectious diseases [31]. The widespread use of herbal remedies and healthcare preparations, such as those described in ancient texts like the Vedas and the Bible has been traced to the occurrence of natural products with medicinal properties [32-33]. There is an increased interest in the application of herbs in fish disease management [34]. This work attempts to find out the efficacy of two traditional herbs Acorus calamus and Indigofera aspalathoides in the treatment of experimentally induced Aeromonas hydrophila infection in Koi carp. These herbs were chosen out of the five herbs that were tested for their anti-microbial property against A. hydrophila In vitro [35-36].
Materials and Methods
Plant Material
A. calamus and I. aspalathoides are widely distributed in tropical and warm temperate regions. These plants are commonly used in folk medicine to treat dermatitis, gastric ulcer, abrasion, lesions and inflammation [37].
Preparation of Plant Extract
The fresh rhizome of A. calamus and stem part of I. aspalathoides were collected and washed under running tap water for 5 minutes; the small hairs of the rhizome were removed, and the parts of the plants were chopped and shade dried for a week to achieve weight constancy. The dried parts were finely powdered in an electric blender and stored in airtight bottles. To obtain herbal residue the powders were extracted with 90% w/w ethanol using a soxhlet apparatus. The ethanol was removed under pressure using a rotary evaporator. The dried crude herbal residues were stored in a dark air tight bottle 4ºC for further Studies [32].
Preparation of Crude Extract for Short Bath Treatment
The known quantity of each plant residue was dissolved in sterile water and then added to the water for short bath treatment so as to contain 2 mg residue/liter.
Aeromonas hydrophila
The reference strain A. hydrophila (MTCC Code No-646) was purchased from the Institute of Microbial Technology (Govt. of India), Chandigarh and maintained in the laboratory under standard conditions. Subcultures were maintained on Tryptone soya agar slopes at 30ºC (Hi Media, Mumbai, India) and routinely tested for pathogenesis [38] by inoculation into fishes. A. hydrophila were incubated in 100ml Muller Hinton broth for 18 hrs at 30ºC the culture was centrifuged at 10,000 rpm for 20min at 4ºC [34]. The supernatant was discarded, and the bacterial pellet was washed with phosphate buffer saline (pH-7.2) and prepared to contain 2.7 ×10³ colony forming unit (cfu/ml) of A. hydrophila as determined using a Neubauer haemocytometer slide [39-40]
Collection of experimental Animal
The test fish koi carp Cyprinus carpio (27.6±4.27 g; 207.2±0.77 mm) were acclimatized in tanks (2000 L capacity; 6×4×4 m) to the laboratory condition for at least 7 days and fed of almost same size (30 to 40 cm) were collected alive in healthy conditions from kadachnenthal fish farm, Madurai, Tamil Nadu during pre-monsoon, monsoon and post monsoon seasons. The fishes were transported from the reservoir in oxygenated bags to the Laboratory and immediately some control fishes transferred into the glass aquaria of 50 liters capacity containing well aerated, unchlorinated ground water for 7 days acclimatization. The other fishes with the active movement were only used for the experimentation. The fishes were screened for any physical damage, disease and mortality. The immobilized, injured, abnormal and dead fishes were discarded immediately.
Experimental Design
A total of 190 fishes were divided into seven groups of 10 each in triplicate as follows: Group a) infected untreated (IU) (10×3=30); Group b) Short bath treatment with A. calamus (rhizome) (IBTAc) (10×3=30) or I. aspalathoides (IBTIa) extract (10×3=30); Group c) fed with a standard diet enriched with each of the herbal (pulverized) powder (IFAc (10×3=30) & IFIa) (10×3=30) and Group d). a combination of the treatments b and c (IBFAc & IBFIa) (10×3=30) and Control (10×1=10).
Experimental of Fish
The koi carp Cyprinus carpio (27.6± 4.27 g; 207.2±0.77 mm) were acclimatized in tanks (2000 L capacity; 6×4×4 m) to the laboratory condition for at least 7 days and fed ad libitum with standard diet twice a day. Before the commencement of the experiment the fish were not fed for two days. The physico-chemical conditions recorded are: temperature 28 ±2ºC, dissolved oxygen 3.4 ml/lit, total alkalinity 52 ppm, total hardness 14.0 N and pH 7.0.
Induction of Infection
The pathogen (2.7 × 10³ in 0.2 ml of sterile distilled water) were administered intramuscularly (0.2 ml) using 20-gauge needle. The control fish were sham injected with plain distilled water. During the entire experimental period that lasted 25 days the fishes were examined daily in the morning and evening to observe the gross pathological changes in the lesion size, formation of epidermis and fresh scales.
Short -Term bath treatment
The fish is dipped into a concentrated phytochemical bath for a period of time, less than 5 minutes.
Feed Enrichment with Herbal Powder
Preliminary studies were made to find out the effective dosage of the herbs for feeding. At 50% level the incorporated diet was not fed by the fishes. At 25% level the fished readily fed on the diet and hence this was chosen for the study. The percentage composition of the standard feed [41] made from the locally available ingredients was modified to enrich the feed with the herbal powders as given below:- Feed–I Ground nut oil cake 27.26 gm, sesame oil cake 27.26 gm, rice bran 11.36 gm, tapioca powder 11.36 gm, fish oil 0.4 ml, vitamin mix 0.1 gm, mineral mix 0.1 gm and Acorus calamus (rhizome) 22.13 gm. Feed - II: Ground nut oil cake 27.26 gm, Sesame oil cake 27.26 gm, rice bran 11.36, gm tapioca powder 11.36 gm, fish oil 0.4 ml, vitamin mix 0.1 gm, mineral mix 0.1gm and I. aspalathoides (stem) 22.13.The powdered rhizome and stem of A. calamus and I. aspalathoides respectively along with the dried and powdered ingredients were thoroughly mixed in and electric blender. The feeds were made into soft dough with water, extruded through a pelletizer (0.2 mm), dried in shade for 15 days and stored in a refrigerator at 4ºC till used. The formulated standard and herbal diets were fed ad libitum twice daily in the morning and evening; the unfed were siphoned out once in the morning and 2/3rd of the water was changed daily.
Blood Samples
Sampling was performed 10hrs after the last feeding by puncturing the caudal puncture [42]. Before collecting blood samples, no anesthetic was applied to fish [43] as it may affect blood parameters and hemolysetissues [44-45]. EDTA and sodium heparin (5000 IU in 1 ml injection) were used as anticoagulants, the former being used for the haematological examination.
Hematology and Clinical Chemistry
The RBC (Red Blood Cell) was counted in 2 × 20 rectangles per sample haemocytometer in Hayem solution. Leukocyte counting was performed by transporting blood sample with a leukocytes pipette into counting chamber and examined for erythrocytes [46] RBC [47] and WBC+ thrombocytes [48] were determined using a Neubauer hemocytometer. Differential white cell and thrombocyte counts were done on blood films stained with Giemsa. For every 1200 erythrocytes counted at random, the number of thrombocytes and the different types of leucocytes was determined on each blood smear.
Determination of Heamatocrit
Hematocrit value was determined by the standard microhematocrit method and expressed in percentage. Duplicate blood samples were loaded into standard heparinized capillary tubes, spun in a microhematocrit centrifuge at 12,000 rpm for 5 min and measured on a microcapillary reader
Determination of Haemoglobin
The haemoglobin count was estimated by acid – Haematin method using Sahli’s haemocytometer. Pure blood was drawn into Sahli’s pipette up to the 0.02 mark. The blood was expelled into a haemometer tube containing 0.1N HCl, up to the mark. After removing the pipette, the content was thoroughly mixed using a stirrer. The mixture was diluted with distilled water by adding few drops at a time with thorough mixing, until the colour of the solution matches with the glass plate of the comparator. The level of the fluid was noted at the lower meniscus. The amount of haemoglobin was directly read in gm%.
Determination of Haematocrit (Ht) or Packed Cell Volume (PCV)
Wintrobe’s apparatus was used in this method and microhaematocrit method was followed due to the limited amount of blood available. RBC (1.090-sp-gr) being heavier than plasma (1.030-sp-gr) get packed towards the bottom of the tube by centrifugal force. The Wintrobe’s tube was filled with blood mixed with anticoagulant up to 10 mark. Blood was centrifuged for 30mts at 3000 rotations per minute. The blood was separated into 3 layers - a tall bottom layer of red cells, a thin middle layer of WBC and top layer of clear plasma. After this the length of the packed RBC column was noted. Haematocrit was calculated as: Ht = L1/L2 × 100 Where L1 = Height of RBC column in mm. L2 = the total length of column (RBC + WBC + plasma) in mm (10) Ht is expressed as %.
Determination of Erythrocyte Indices
From the values of haemoglobin content, haematocrit and total erythrocyte count, erythrocyte indices were calculated using the respective formula [49].
A. Mean Corpuscular Volume (MCV)
It represents the average of individual erythrocyte in cubic microns (µ3/fL) and computed by the formula, MCV = Ht%/RBC in million/mm 3 × 10
Mean Corpuscular Haemoglobin (MCH)
MCH represents the average weight of haemoglobin in pictograms (pg) and calculated by the formula, MCH = Hb%/RBC in million/mm 3 ×10
Mean Corpuscular Haemoglobin Concentration (MCHC)
MCHC is the average haemoglobin concentration per 100 ml of packed erythrocytes in percent and computed by, MCHC = Hb%/Ht% × 100
Plasma Biochemistry
The blood samples were centrifuged at 12000 g for 3 minutes at room temperature (28±2ºC) to separate the plasma; this was used to determine the glucose and cholesterol by colorimetric method with commercial kits (sigma diagnostics). The total proteins were measured using bovine serum albumin as standard [50].
Assays to Measure Biochemical and Non-specific Immunological Responses:
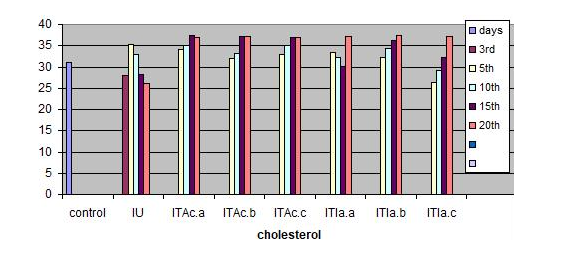
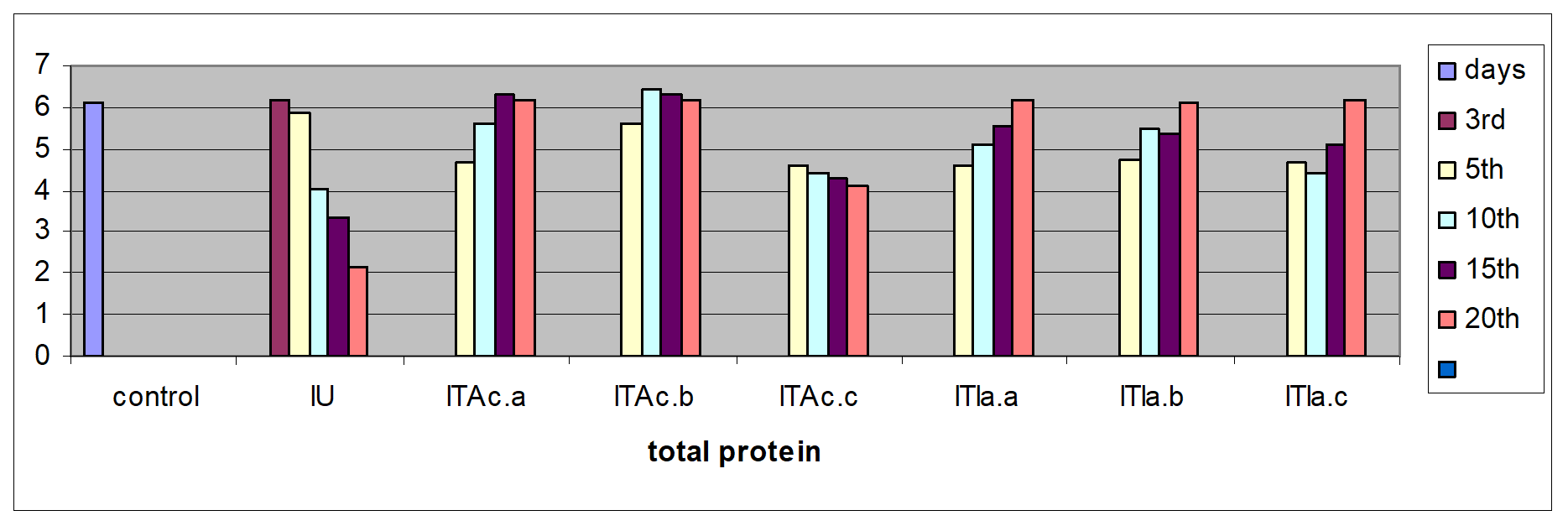
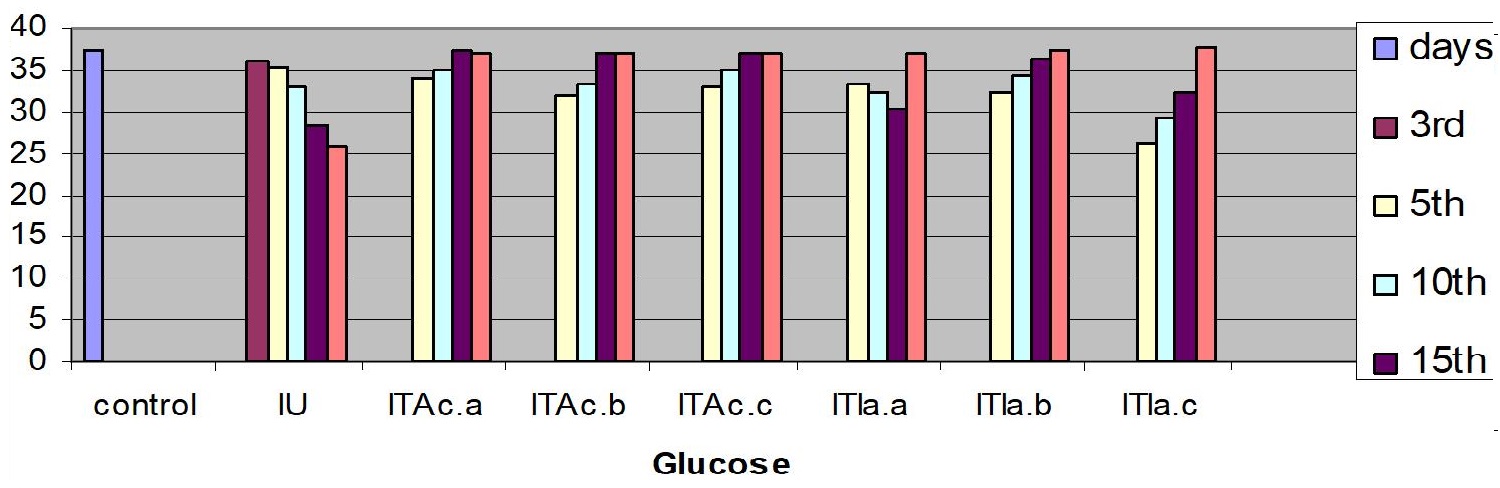
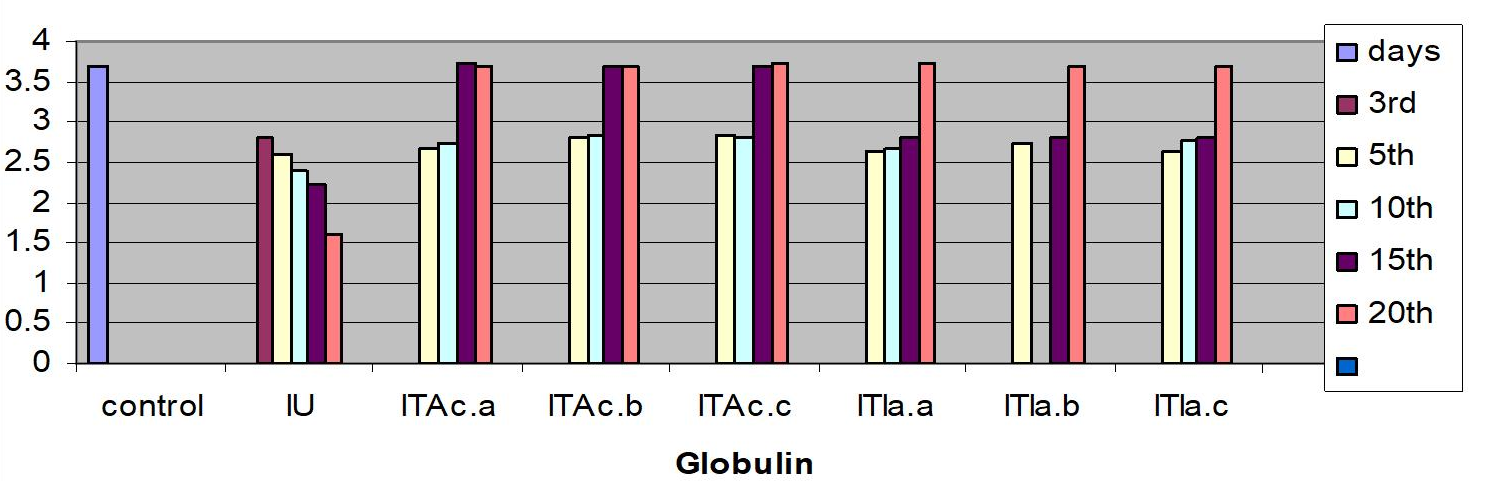
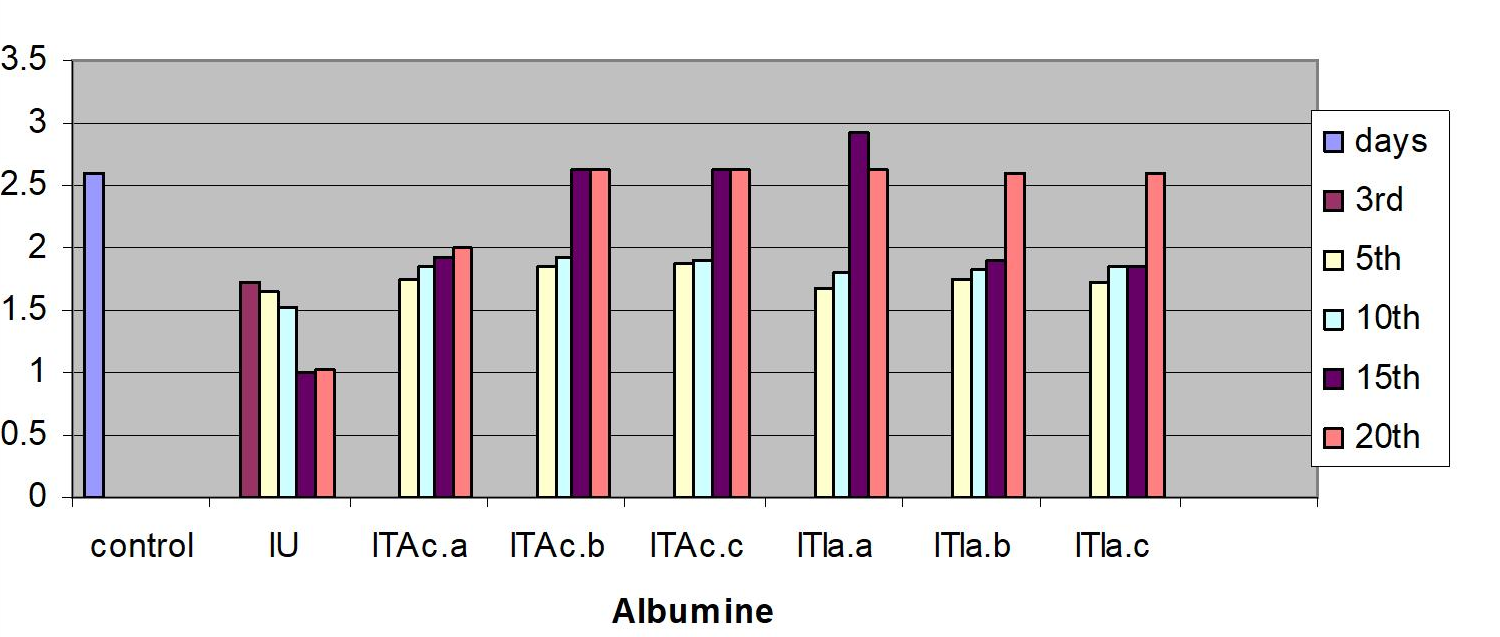
Measurements were performed for glucose concentration, lysozyme activity, total protein and total cholesterol, and respiratory burst activity of blood cells. The glucose concentration in fish blood is expected to increase four hours after stress exposure [51-52]. It was measured immediately after bleeding by a kit of Haemo-Glukotest 20-800 R (Reflolux S, Boehringer Manheim). Its activity and concentration in the plasma is measured by spectrophotometry. Lysozyme is an important enzyme in the blood that actively lyses bacteria. Total protein and total cholesterol were measured according to established procedures [53-54], respectively). Total protein was only measured at the base line level. The components measured by the auto analyzers are listed in Figure. Globulin levels were determined indirectly by subtracting the measurement of albumin from total protein. Protein fraction levels were determined by agarose gel electrophoresis following the procedure described by [55].
Statistical Analysis
All replicates were used for the calculation of mean values. Differences in haematological parameters between exposure times has been subjected to statistical analysis using students test and ANOVA, to manifest the variations in comparison with control. The variations were reported in their significant levels. The hematological parameters were expressed as mean ± standard deviation Data are presented as mean ±SD for 10 fish per group. The data were analyzed using the student’s t-test to compare the differences in values between infected, herbal treated and the normal fish using the statistical package COSTAT® for MS Windows TM.
Results
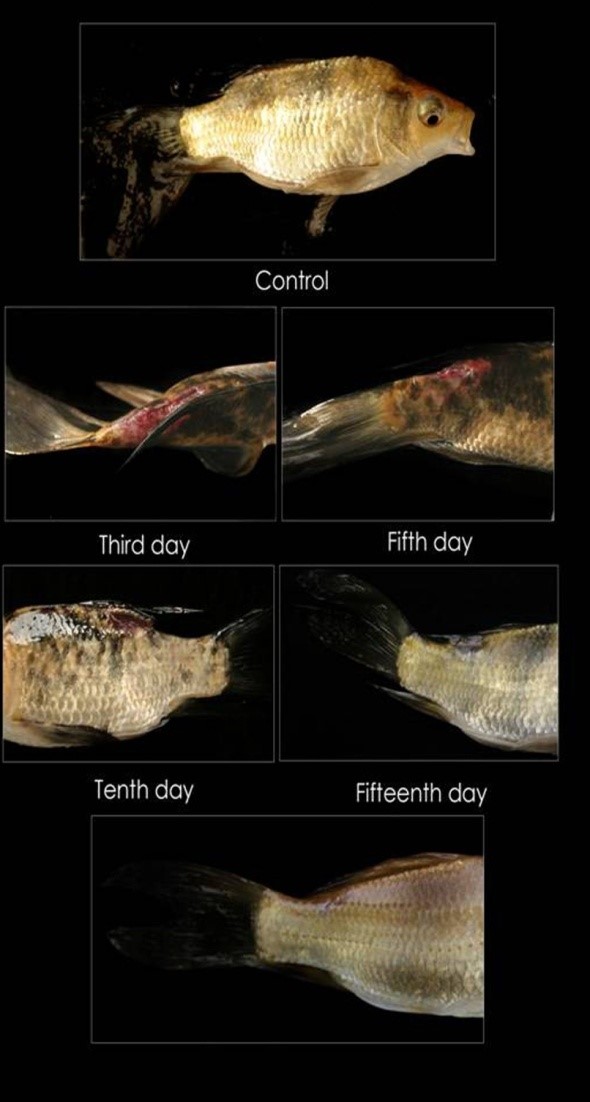
Blood is a patho-physiological reflector of the whole body and therefore, blood parameters are important in diagnosing the structural and functional status of the animal exposed to toxicants. The circulatory system of fish is in close association with the external environment and with every tissue. It is sensitive to foreign stimuli and reflects the homeostasis of the animal. Thus, haematological studies help to check the systemic responses during diseased conditions. The effect pathogenic infection on various haematological parameters of fish Koi carp studied, and results are included here. In the treated groups all the fish survived has during the entire experimental period of 25 days. However, in the infected untreated group about 20% started succumbing daily from 20± 2.00 days onwards with a 50% cumulative mortality on 25days. The inoculated fishes showed clinical signs of Aeromonas infection; in the infected-untreated group the lesion size grew from healing on the third day to scale formation on the 25 days; in the treated groups the lesion gradually decreased in size with the formation of epidermis and scales (Table 1 and Figure 1). The changes in hematological parameters like leukocyte, erythrocyte count, thrombocyte, hematocrit, hemoglobin and various indices significantly (P<0.05) differed from the control (Table 2a and b).
Table 1: Healing of lesion in Koi carp treated with various Indian medicinal plants to determine the potent herbs *Epidermis formed; **Appearance of rudimentary scales; ***Complete formation of scales Each value is a mean ± SD of 5 observations. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Figure 1: Cyprinus carpio is healing of lesion from 1.72 ± 5.9 mm on Control (infected Day), the third day, the fifth day, tenth day and fifteenth day in the infected fish as a function of time (days) after short term bath (2 mg/l; 10 minutes/day) treatment with phytotherapuzed with A. calamus. |
|
The WBC (white blood cell) and RBC levels of the infected-untreated fish initially increased (P<0.05) from the control values on the 5th, 10th, 15th and 20th, day. After 20th day the WBC level in infected fish significantly increased (P<0.05) to a maximum. The WBC and RBC level in treated animals of groups IBA.c.& IBI.a., IBFA.c.& IBFI.a., did not vary (P>0.05) between 10th and 15th day and attained near normal value (P>0.05) from 20th day onwards.
In the infected untreated groups MCH, MCHC, and LYMs levels decreased (P<0.05) whereas the hemoglobin level decreased slightly (P>0.05) on 3rd, 5thand 10th day (Table 1). However, in the infected untreated group a significant decrease in Hb content and MCH was observed (P < 0.05). The levels of plasma protein, albumin, globulin, cholesterol, plasma glucose in the infected fish decreased from the control value (P>0.05); the treated fish registered an increase (P>0.05) when compared to the infected untreated group (Table 3).
Discussion
Blood tissue of fish gives clue about physical condition of fish [56]. In recent years, blood parameters have been commonly used to observe and follow fish health [57]. Increase in glucose concentration is a secondary response to stress, and the level of increase is a measurement for stress response. The aquaculture environment exposes the fish to a regime of repeated acute stress, which has deleterious effects on growth, reproduction and the immune response [58]. In most of the infected fishes the homeostatic processes are extended beyond the normal value due to stress [59-60]. Spurt in the WBC level in response to an infection is the first line defense by any organism; fish is no exception [61]. The increase in WBC level is consistently high during the entire period of infection at Oncorhynchus kisutch [62] and Carassius auratus [63]. In the present study also, the WBC level of infected koi carp consistently increased (P<0.05) from the control value in the on 5th, 10th, 15th and 20th day and reached the maximum on 25th day. In the treated fish it decreased from 5th to the 25th day (P>0.05).Decreased RBC counts indicate that RBC is being destroyed by the leucocytosis activity with subsequent erythroblastosis in erythrocytic anemia [64-65]. This trend is also reported in various fishes infected with epizootic ulcerative syndrome (EUS) [61]; for instance pearl spot fish Etroplus suratensis when infected with EUS becomes anemic [66]. In the treated koi carp IBFA.c.20 and IBTI.a.20 these values were restored to the normal value (Table 3 and 4).
Notes: A. c. - A. calamus Table 3: C. carpi changes in hematological parameters (mean ± SE; n = 0) in infected untreated (IU) against control (C) fish phytotherapuzed with A. calamus at 5 days interval. |
Notes: A. c. - A. calamus Not significant (P > 0.05), * Significant (P < 0.05), ** Highly significant (P < 0.01) Table 4: Significant level of hematological parameters in different groups treated with A. calamus |
The hematocrit value of the infected group slightly increased (P>0.05) between up to the 10th day; subsequently it increased significantly up to 25thday (P<0.05); such a gradual increase in hematocrit can occur in response to induced stress like hypoxia [67]. After treatment the values were restored to near normal in IBFI.a.20 and IBTA.c.20 groups.
Hb contents decreased in infected untreated group more significantly than in the control group. Anemia associated with A. hydrophila infection is adduced to the ability of the pathogen to produce protease enzymes that can haemolyse erythrocytes. The incidence of decreased erythrocyte count with experimentally induced infection in Cyprinus carpio is also reported [34]. Increased neutrophil, monocyte and basophile counts indicate a strong phagocytosis; lymphocytes and erythrocytes [68]. Salmo gairdneri infected with A. hydrophila showed an elevated Hct [69], plasma glucose level and increased WBC counts [70]. C. carpio infected with A. hydrophila had a marked decrease in RBC counts, Hb and plasma protein with resultant anemia [71].
In this study, the LYMs of infected treated group increased beyond the normal. Generally, the LYM affords defense against pathogens since the maximum number of leukocyte and macrophages exhibit enhanced phagocyte activity [61]. This study establishes that like Oscimum sanctum in Oreochromis mossambicus [72], traditional herbs like A. calamus and I. aspalathoides also bestow immunostimulatory effect since there is a significant increase in the number of activated LYMs from 5th day of treatment to the end. Treatment with Osmium sanctum results in changes in total and differential leucocytes conferring a state of non-specific increased resistance in animals and man [73-74].
Hence in fish disease management homeopathic or ayurvedic drugs can be an alternative choice. For example, in the ulcerative disease management in fishes, chemotherapy with copper sulphate, potassium permanganate and common salt solution did not yield positive results; however, significant recovery was achieved with intramuscular injection of the homeopathic drugs (i.e.) heaper Sulphur and arnica spray [75].
The serum albumin and globulin levels did not increase (P>0.05) (Fig 1c & d) in infected koi carp. In stressed Ictalurus punctatus also the plasma protein values did not vary significantly from the healthy fish [76]. A decrease in cholesterol, and mainly total serum protein as observed in this work has also been reported in common carp infected with spontaneous spring viremia [77] and in Salmo trutta and Salmo salar diseased with ulcerative dermal necrosis [78]. The increase in the serum protein and globulin levels induces a strong innate immune response in fish [79] in the present study the affected total protein and globulin levels were restored to near normal in all the treated groups indicating that the treatment with A. calamus and I. aspalathoides act as a immunostimulatory (Figure 2).
|
a. Total protein (g.1-1 )
b. Glucose (mmol.1 -1)
c. Globulin (%)
d. Albumin (%)
Figure 2: C. carpio changes in the biochemical indices of control, infected-untreated (IU), infected - A. calamus (IB Ac), and infected - I. aspalathoides treated (IB Ia), groups for 25 days (Fig1. a, b, c, d, e and f). |
Many Indian ayurvedic books report the wound healing properties of A. calamus and I. aspalathoides [80]. The wound healing property of A. calamus has been documented [81]. Treatment with I. aspalathoides heals the lesion in albino mice [82-83]. Dip treatment with aqueous neem leaf extract (1%) could heal the ulcerative dermatitis of Cyprinus carpio infected with A. hydrophila within 30 days; C. carpio infected with A. invadans within 25 days, restoring the hematological and biochemical parameters [34,63]. The present study shows that phytotherapy with A. calamus heals the muscular dermatitis and restores the affected hematological parameters within 20 days of short bath treatment (2mg/lit) for 10 minutes daily. Further work is underway: a) to find out whether pretreatment of koi carp with the herb can trigger immunostimulation and can be used as a preventive measure to resist infection when challenged with A. hydrophila and b) to isolate the active compounds from these extracts.
Notes: I.a. - I. aspalathoides Table 5: C. carpio: changes in hematological parameters (mean ± SE; n = 10) in infected untreated (IU) against control (C) fish phytotherapuzed with at 5 I. aspalathoides days interval. |
Table 6: Significant level of haematological parameters in different groups treated with I. aspalathoides Notes: I.a. - I. aspalathoides; NS: Not significant (P>0.05), * Significant (P<0.05), ** Highly significant (P < 0.01) Notes: I.a. - I. aspalathoides; NS: Not significant (P>0.05), * Significant (P<0.05), ** Highly significant (P < 0.01) |
References
- Kutty MN (2003) Sustainable Aquaculture-A comparative view of freshwater prawn and marine shrimp Freshwater prawns. In International symposium Souvenir, Kerala Agriculture University, Kochi, India pp14-20.
- Kestemont P (1995) Different systems of carp production and their impacts on the environment. Aquaculture 129: 347-372.
- Davis JF, Hayasaka SS (1983) Pathogenic bacteria associated with cultured American eels, Anguilla rostrata Le Sueur. J Fish Biol 23: 557-564.
- Jeney Z, Jeney G (1995) Recent achievements in studies on diseases of common carp (Cyprinus carpio L.) Aquaculture 129: 397-420.
- Grondel JL, Nouws, JFM, De Jong M, Schutte AR, Driessens F (1987) Pharmacokinetics and tissue distribution of oxytetracycline in carp, Cyprinus carpio L., following different routes of administration. Journal of Fish Diseases 10: 153-163.
- Moral C H, del Castillo E F, Fierro P L, Cortés A V, Castillo J A, Soriano A C, Carrasco G N (1998) Molecular characterization of the aeromonas hydrophila aroa gene and potential use of an auxotrophicaroa mutant as a live attenuated vaccine. Infection and Immunity 66: 1813-1821.
- Palumbo S, Auya C, Steima G 1992 Aeromonas hydrophila group; In compendium of methods for microbiological Examination of food. In; Vanderzant, cand splitstoesser. F. (Eds) Washington APHN. 497-51.
- Gopalakrishnan V (1961) Observation on infectious dropsy of Indian major carps and its experimental induction. JSIR 20 : 357-358.
- Swann L, White M R (1991) Diagnosis and treatment of" Aeromonas hydrophila" infection of fish. Aquaculture Extension, Illinois-Indiana Sea Grant Program.
- Rukyani A (1994) Status of epizootic ulcerative disease in indonesiaIn; Roberts, RJ Cambell, B Mac, rea TH. In Proceedins of ODA Regional seminar on epizootic ulcerative syndrome. Aquatic Animal health research Institute, Bangkok (Vol. 13, pp. 25-27).
- Stoskopf, M.K. 1993. Fish medicine WB. Saunders Co. Philadelphia. 473- 474.
- Mitchell A J, Plumb J.A (1980) Toxicity and efficacy of Furanace on channel catfish Ictalurus punctatm (Rafinesque) infected experimentally with Aeromonas hydrophila. Journal of Fish Diseases 3: 93-99.
- Rath, R.K., 2000. Fresh water Aquaculture, 2nd Eds. Scientific Publishers (India), Jodhpur, India. 257.
- Service, R.F. 1995. Antibiotics that resist resistance. Sci. 270; 724-727
- Blaxhall P C (1972) The haematological assessment of the health of freshwater fish: a review of selected literature. Journal of fish biology 4: 593-604.
- Wedemeyer G A, Gould R W,Yasutake W T (1983) Some potentials and limits of the leucocrit test as a fish health assessment method. Journal of Fish Biology 23: 711-716.
- Golovina, N.A., Trombicky, I.D., 1989. Haematology of Pond Fish. Kishinev, Shtiinca, p. 158.
- Zhiteneva, L., Poltavceva, T.G., Rudnickaja, O.A., 1989. Atlas of normal and pathological cells in the blood of fish. Rostov-on-Don, p. 112.
- Bielek E, Strauss B (1993) Ultrastructure of the granulocytes of the South American lungfish, Lepidosiren paradoxa: Morphogenesis and comparison to other leucocytes. Journal of morphology 218: 29-41.
- Golovina N A (1996) Morpho-Functional Characteristics of the blood of fish as objects of aquaculture. Doctoral thesis. Moscow 53.
- Luskovaá V (1997) Annual cycles and normal values of hematological parameters in fishes. 31: 70
- Vosylien? M Z (1999) The effect of heavy metals on haematological indices of fish (survey). Acta Zoologica Lituanica 9: 76-82.
- Hrubec T C, Smith S A, Robertson J L (2001) Age-related changes in hematology and plasma chemistry values of hybrid striped bass (Morone chrysops× Morone saxatilis) Veterinary Clinical Pathology 30: 8-15.
- Gabriel U U, Ezeri G N O, Opabunmi O O (2004) Influence of sex, source, health status and acclimation on the haematology of Clarias gariepinus (Burch, 1822). African Journal of Biotechnology 3(9).
- Campbell T W (2004) Haematology of lower vertebrates in: proceedings of the 55th Annual meeting of the American college of veterinary pathologists (ACUPC). ASVCP, USA.
- Van Vuren J H (1986) The effects of toxicants on the haematology of Labeo umbratus (Teleostei: Cyprinidae). Comparative biochemistry and physiology. Comparative pharmacology and toxicology 83: 155-159.
- Hancock R E W, Knowles D 1998 Are we approaching the end of the antibiotic era? Editorial over view. Current Opinion in Microbiolog 1; 493-494.
- Bax, R., Mullan, N. & Verhoef, J. 2000. Temillennium drugs–the need for and developed new antibacterial agent. Journal Antimicrobials.16; 51-56.
- Knowles D J (1997) New strategies for antibacterial drug design. Trends in microbiology, 5: 379-383.
- Breithaupt H (1999) The new antibiotics. Nature biotechnology 17: 1165.
- Vaidyarathnam, P.S.V. 1995. Indian medicinal plants, Aryavaidya sala, kottakkal, orient Longman Ltd. Chennai.
- Nair R, KALARIYA T, Chanda S (2005) Antibacterial activity of some selected Indian medicinal flora. Turkish Journal of biology 29: 41-47.
- Stiffness M, Douros J (1982). Current status of the NCL plants and animals product program. J Nat Prod.45: 1-45.
- Harikrishnan R, Rani M N,Balasundaram C (2003) Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 221: 41-50.
- Bhuvaneswari R A, Balasundaram C (2006) Traditional Indian herbal extracts used in vitroagainstgrowthofthepathogenicbacteria–Aeromonas hydrophila. Islamic Journal of Aquaculture 58; 89-96.
- Rajagopal B, Narasimman M, Saravana B P (2016) Screening of selected medicinal plants from Tamil Nadu, South India for antibacterial activity against selected fish pathogenic microbe. Research Journal of Biotechnology 11: 46-54.
- Vaidyarathnam, P.S.V. 1995. Indian medicinal plants, Aryavaidya sala, kottakkal, orient Longman Ltd. Chennai.
- Joseph S W, Carnahan A (1994) The isolation, identification, and systematics of the motile Aeromonas species. Annual Review of Fish Diseases 4: 315-343.
- Yadav M, Indira G, Ansary A (1992) Cytotoxin elaboration by Aeromonas hydrophila isolated from fish with epizootic ulcerative syndrome. J Fish Dis 15: 183-189.
- Sundaraj, T. 1997. Microbiology laboratory manual, Ed. Dr. A.L.M.P-G. Institute of basic science, Uni. of Madras. Chennai.
- New M B, Tacon A G, Csavas I (Eds.) (1995) Farm-made aquafeeds. Food & Agriculture Org 343; 41- 143.
- Kocabatmaz, M.H. & Ekingen, G. 1982. Degioik tur baliklarda kan ornegi alinmasi ve hematolojik metotlarin standardizasyonu. Doga Bilimler Dergisi.2; 149-159.
- Ezzat A A, Shabana M B, Farghaly A M (1974) Studies on the blood characteristics of Tilapia zilli (Gervais) I. Blood cells. J Fish Biol 6: 1-12.
- Hoffman, G.L. 1977. Methods for the diagnosis of fish discapet. Fish forming experiment station U.S.A. Fish and Wilt Fish Service, Statpart.
- McKnight, I.M.A. 1966. Hematological study on the mountain whitefish. Popium willasemi South. Journal of Fisheries Research. 23; 45-64
- Blaxhall P C, Daisley K W (1973) Routine haematological methods for use with fish blood. J fish biol 5: 771-781.
- KAPLOW L S (1955) A histochemical procedure for localizing and evaluating leukocyte alkaline phosphatase activity in smears of blood and marrow. Blood 10: 1023-1029.
- Nutt M P,Herrick C A (1952) A new blood diluent for counting erythrocytes and leucocytes of chickens. Poult Sci 31: 735-738.
- Dacie J V, Lewis S M (1975) Measurement and calculation of size of red cells, practical haematology. Churchill Livingstone, London, 40-43.
- Lowry O H, Rosebrough N J, Farr A L, Randall R J (1951) Protein measurement with the Folin phenol reagent. J biol chem 193: 265-275.
- Vijayan M M, Pereira C, Grau E G, Iwama G K (1997) Metabolic responses associated with confinement stress in tilapia: the role of cortisol Comparative Biochemistry and Physiology Part C: Pharmacol, Toxicol and Endocrinol 116: 89-95.
- Melamed O B, Timan R R, Avtalion and E J Noga 1999 Design of a stress model in the hybrid bass (Morone saxatilis x Morone Chrysops). Isr. J. Aquac. – Bamidgeh 51: 10-16.
- Doumas B T (1975) Standards for total serum protein assays—a collaborative study. Clin Chem 21: 1159-1166.
- Allain C C, Poon L S, Chan C S, Richmond W F P C, Fu P C (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20: 470-475.
- ?ehulka J (1993) Erythrodermatitis in Carp (Cyprinus carpio L.): An Electroforetic Study of Blood Serum Protein Fraction Levels. Acta Veterinaria Brno 62: 187-197.
- Ramaswamy M, Reddy T G (1978) A comparative study of hematology of three air-breathing fishes. Proceedings of the Indian Academy of Sciences-Section B. Animal Sciences 87: 381-385.
- Bhaskar B R,Rao K S(1985) Some haematological Parameters of tarpon, Megalops cyprinoids (Broussonet) from Visakhapatham harbor. Matsy 11: 63-69.
- Pottinger T G, Carrick T R (1999) A comparison of plasma glucose and plasma cortisol as selection markers for high and low stress-responsiveness in female rainbow trout. Aquacult 175: 351-363.
- Pickerinng, A.D. 1981. Introduction: The concept of biological stress.In: Pickering, A.D.(Ed.), stress and fish. Academic Press, New York. 1-10.
- Catton W T (1951) Blood cell formation in certain teleost fishes. Blood 6: 39-60.
- Scharperculeus, W. 1991. Textbook of Fish diseases. 1; 75-102.
- Haney D C, Hursh D A, Mix M C, Winton J R 1992 Physiological and hematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J Aquat Anim Health 4; 48-57.
- Harikrishnan, R., Balasundaram, C. & Bhuvaneswari, R. (2005). Restorative effect of Kocabatmaz, M.H. and Ekingen, G. 1982. Deiþik tür baliklardaAzadirchata indica aqueous extract dip treatment on haematological parameter change in Cyprinus carpio (L) experimentally infected with Aphonomyces invadas fungus. J Appl Ichthyol. 21; 410–413.
- Steinhagen D, Kruse P, Körting W (1990) Some haematological observations on carp, Cyprinus carpio L., experimentally infected with Trypanoplasma borreli Laveran Mesnil, 1901 (Protozoa: Kinetoplastida). J Fish Dis 13: 157-162.
- Goel K A, Awasthi A K, Tyagi S K (1981) Comparative haematological studies in some fresh water Indian fishes. Zeitschrift für Tierphysiologie Tierernährung und Futtermittelkunde 46: 202-206.
- Pathiratne A, Rajapaksha W(1998) Haematological changes associated with epizootic ulcerative syndrome in the Asian Cichlid fish. Asian Fisheries Science 11: 203-211.
- Holeton G F, Randall D J (1967) The effect of hypoxia upon the partial pressure of gases in the blood and water afferent and efferent to the gills of rainbow trout. J Exp Biol 46: 317-327.
- Lehmann J, Stuerenberg F J, Mock D (1989) Changes in the haemogram of the rainbow trout (Salmo gairdneri) following an experimental infection with a strain of Aeromonas salmonicida salmonicida. Fish health protection strategies 259-284.
- Swift D J, Lloyd R (1974) Changes in urine flow rate and haematocrit value of rainbow trout Salmo gairdneri (Richardson) exposed to hypoxia. J Fish Biol 6: 379-387.
- Peters G, Faisal M, Lang T, Ahmed I (1988). Stress caused by social interaction and its effect on susceptibility to Aeromonas hydrophila infection in rainbow trout Salmo gairdneri. Dise. Aquatic Organ.4; 83–89.
- Takahashi Y (1984) Appearance mechanisms of haematological symptoms of the aeromonas disease in carp [Cyprinus carpio]. J. Shimonoseki Uni. Fishery 32; 67–74.
- Venkatalakshmi S, Michael R D (2001) Immunostimulation by leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). J. Aquatic Tropic 16; 1-10.
- Sembulingam K, Sembulingam P, Namasivayam A (1999) Effect of Ocimum Sanctum Linn On Changes In Leucocytes Of Albino Rats Induced By Acute Noise Stress. Indian J Physiol. Pharmacol 43: 137-140.
- Archana R, Namasivayam A (2000) Effect of Ocimum sanctum on noise induced changes in neutrophil functions. J Ethno Pharmacol 73: 81-85.
- Mitra S D, Varshney P K (1991) Use of Homeo drug for curing ulcerative syndrome in fishes. J Indian Fish Assoc 21: 55-56.
- Scott A L, Rogers W A (1980) Histological effects of prolonged sublethal hypoxia on channel catfish Ictalurus punctatus (Rafinesque). J Fish Dis 3: 305-316.
- ?ehulka J (2000) Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquacul. 190; 27–47.
- Mulcahy M F (1969) Serum protein changes in UDN-infected Atlantic salmon a possible method of diagnosis. J Fish Biol 1: 333-338.
- Wiegertjes G F, Stet R M, Parmentier H K, van Muiswinkel W B (1996) Immunogenetics of disease resistance in fish: a comparative approach. Develop Compar Immunol 20; 365–381.
- Anonymous. 1956. Sabentha Vaithiyamuraikal. Ayurvedic book, Sarashwathi Mahal Tanjore.
- Rastogi, Mehrotra (1990) Indian medicinal plants. 1, 8.
- Amala Bhaskar E, Ganga N, Arivudainambi R, Santhanam G (1982) Anti-inflammatory activity of Indigofera aspalathoides Vahl Indian J Med Res 76: 115 -128.
- Rajkapoor, Jayakar B, Murugesh N (2004) Antitumor activity of Indigofera aspalathoides on Ehrlich ascites carcinoma in mice. Indian J Pharmacol 36: 38-40.
 LOGIN
LOGIN REGISTER
REGISTER.png)

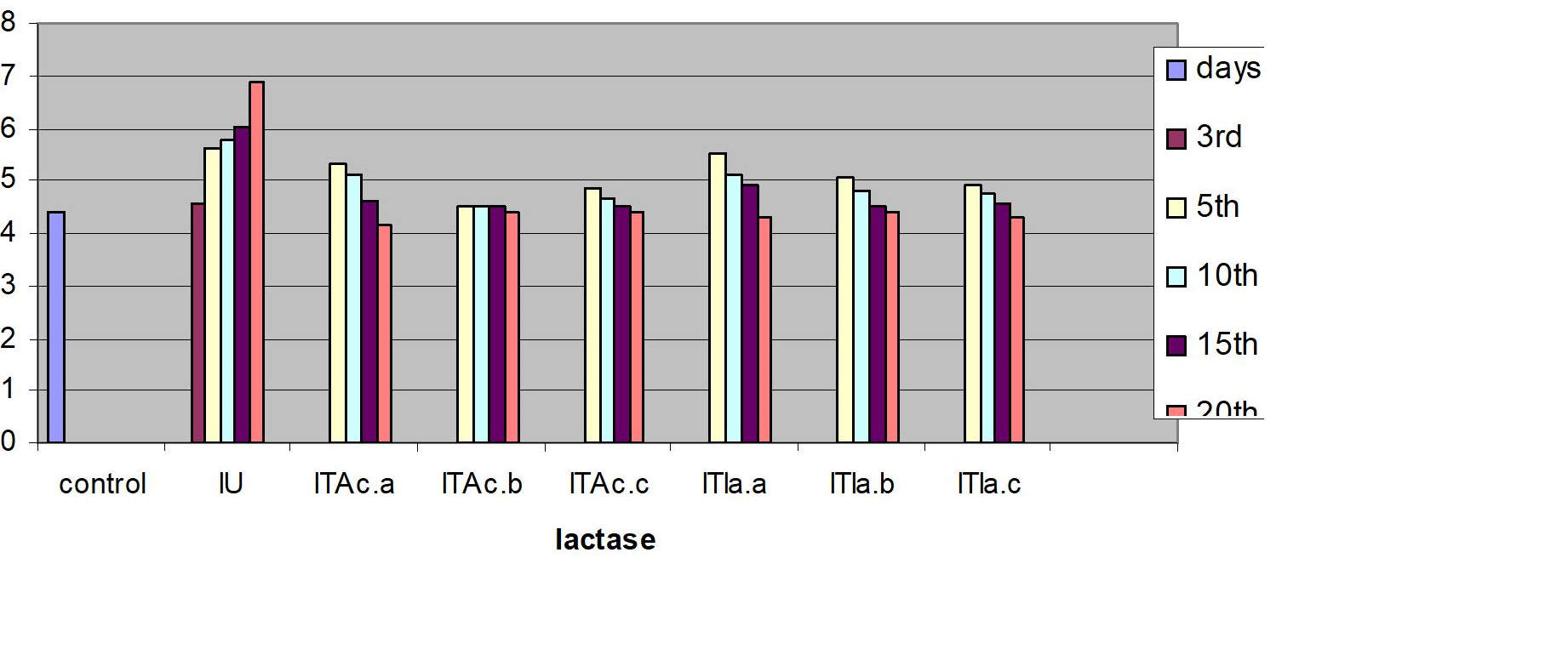 e. Lactase (mmol.1 -1)
e. Lactase (mmol.1 -1)